Deposition Date
2025-09-15
Release Date
2025-12-03
Last Version Date
2025-12-03
Entry Detail
PDB ID:
9Y9W
Keywords:
Title:
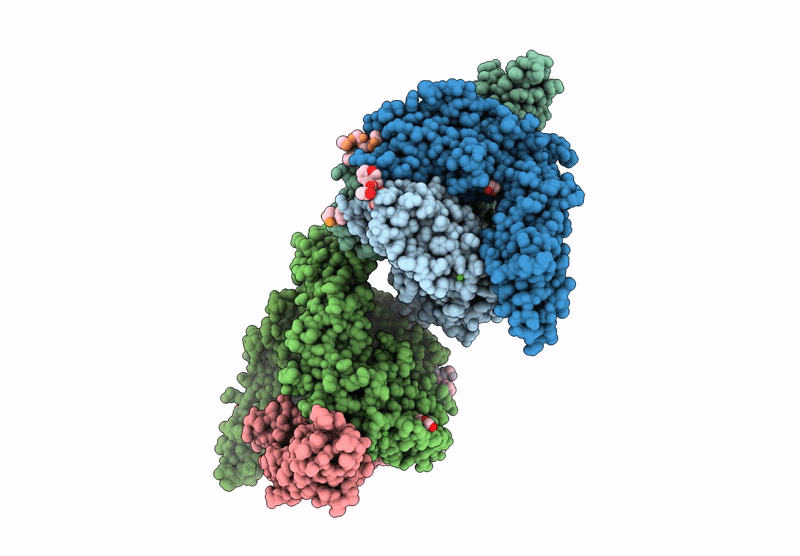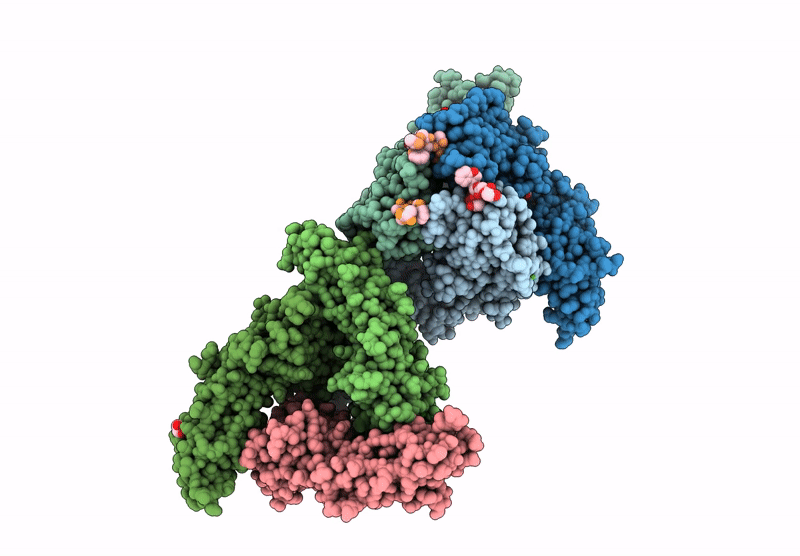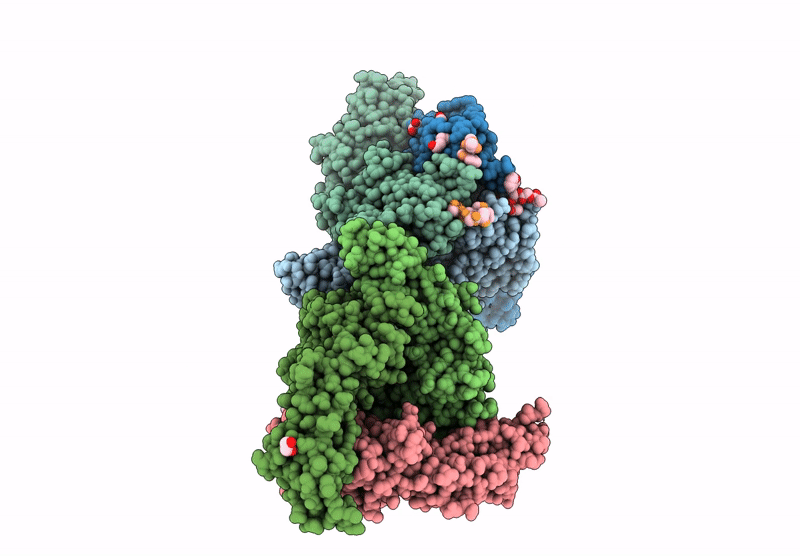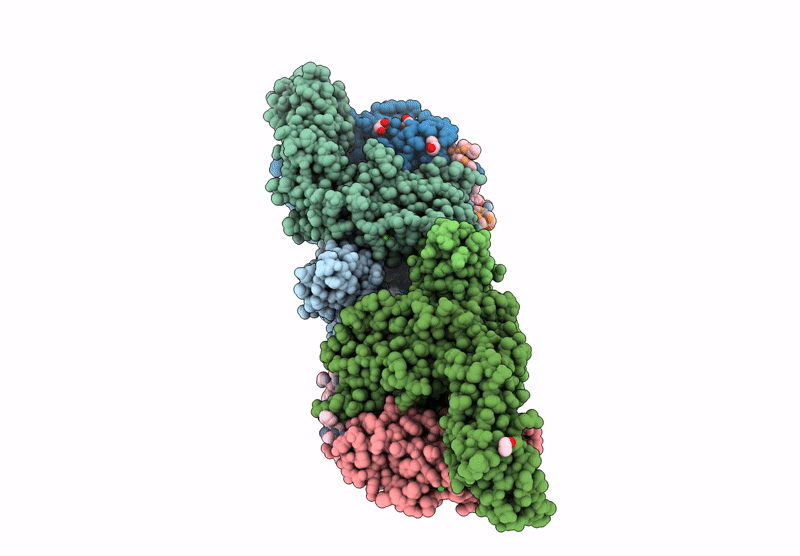
Vibrio cholerae protein FrhA peptid-binding domain and adjacent split domain (S1127-F1439) in complex with peptide AGWTD X-ray crystallography structure
Biological Source:
Source Organism(s):
Vibrio cholerae O395 (Taxon ID: 345073)
Vibrio cholerae (Taxon ID: 666)
Vibrio cholerae (Taxon ID: 666)
Expression System(s):
Method Details:
Experimental Method:
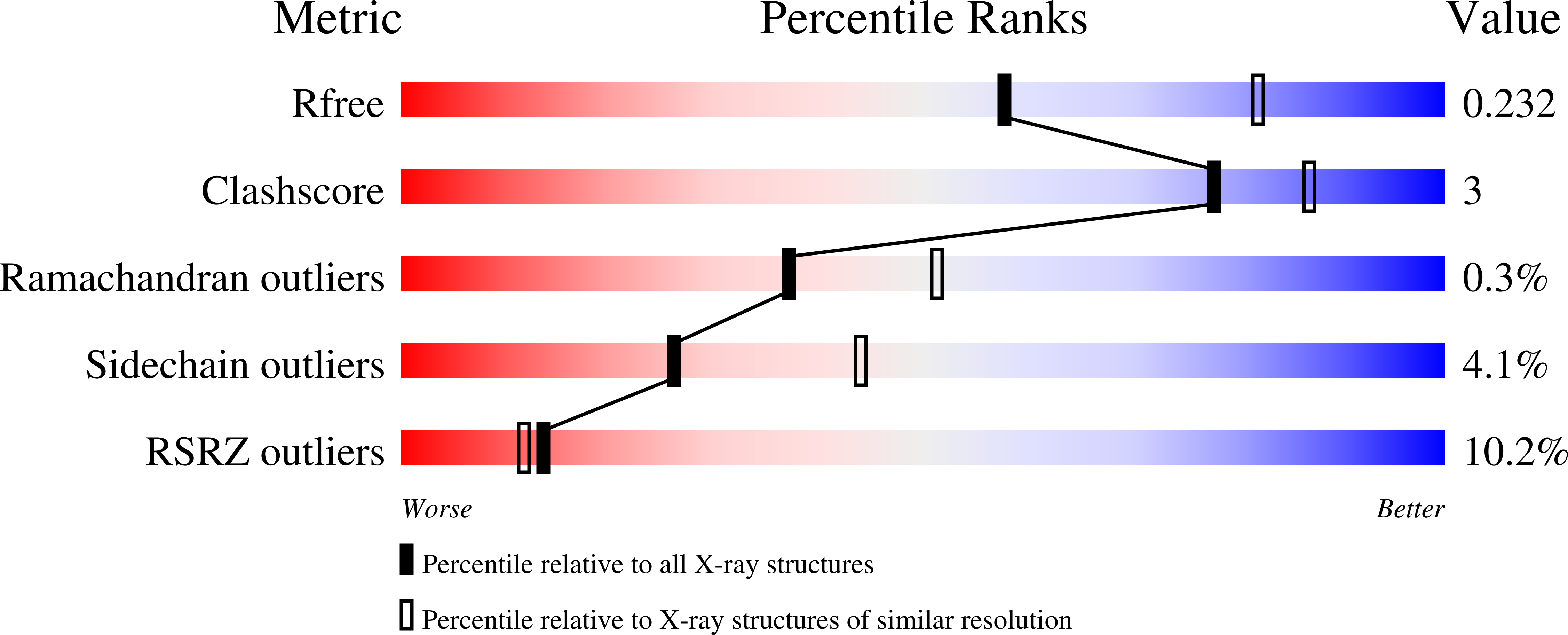
Resolution:
2.40 Å
R-Value Free:
0.23
R-Value Work:
0.22
R-Value Observed:
0.22
Space Group:
P 1