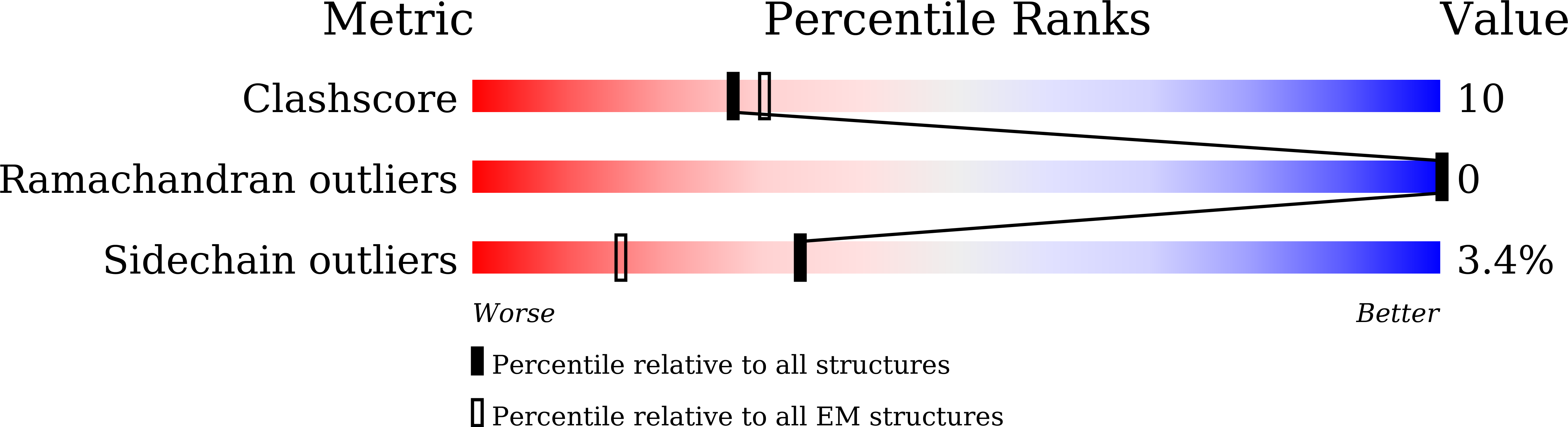
Deposition Date
2025-04-06
Release Date
2025-06-25
Last Version Date
2025-06-25
Entry Detail
PDB ID:
9UD3
Keywords:
Title:
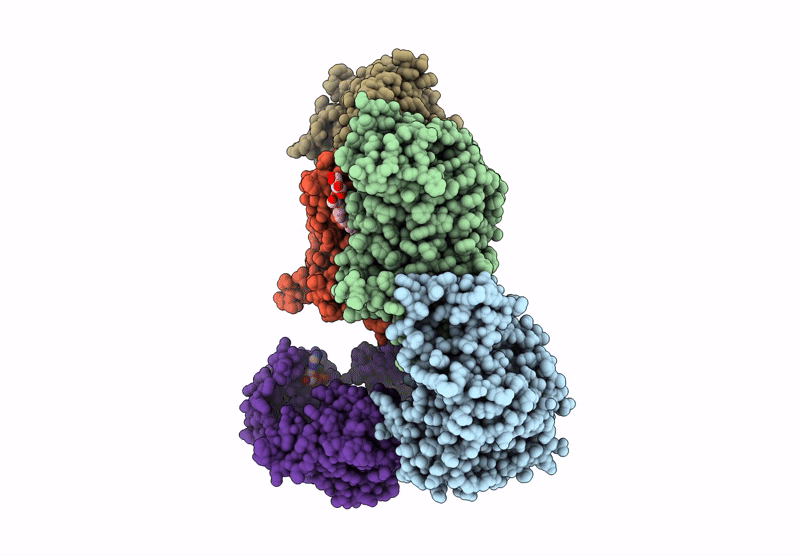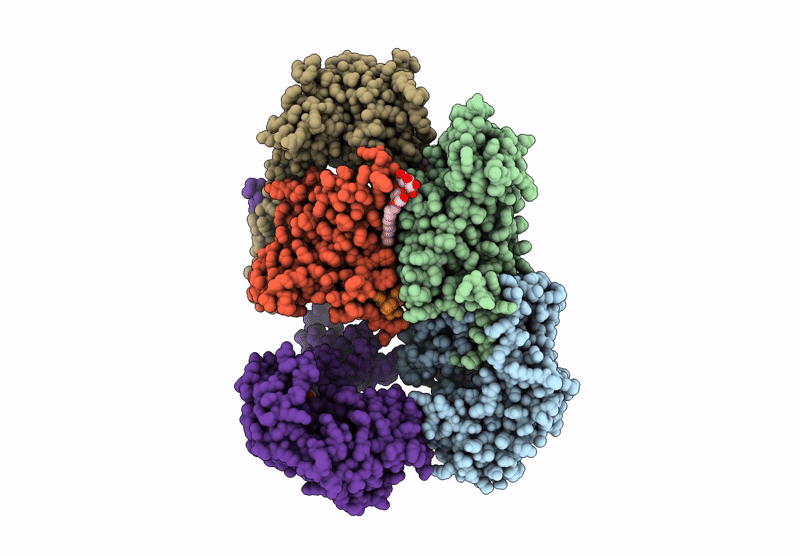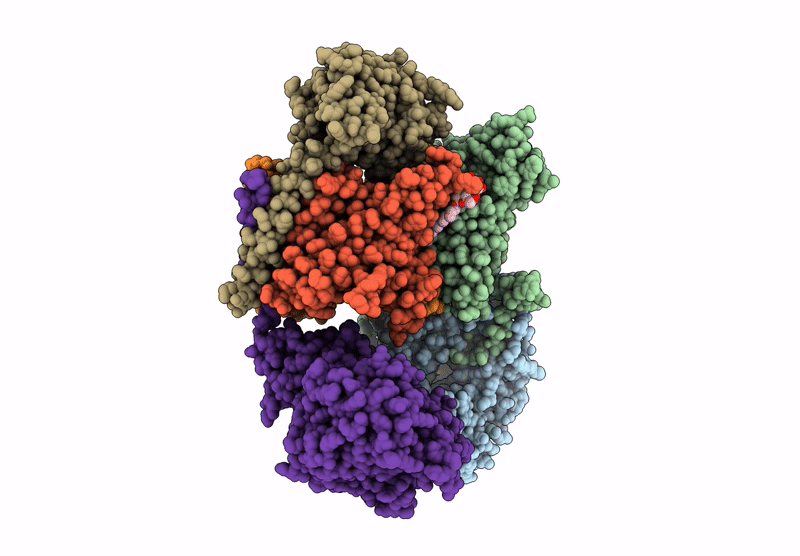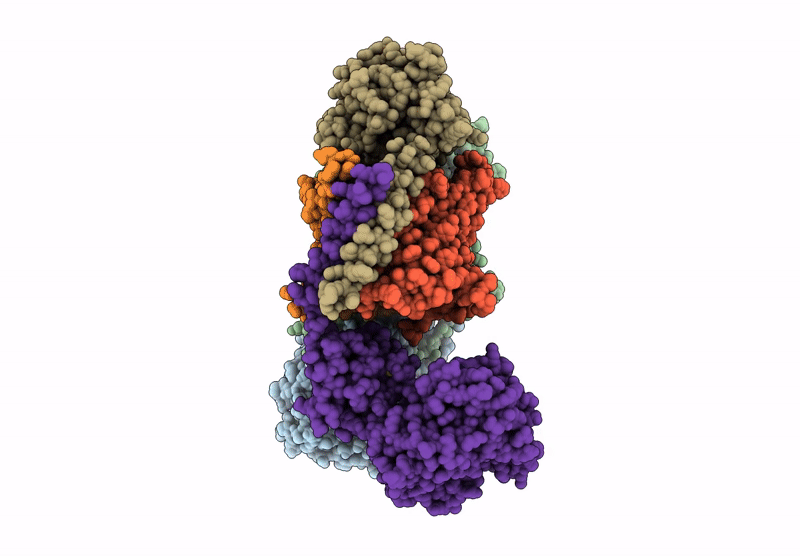
Cryo-EM structure of Na+-translocating NADH-ubiquinone oxidoreductase NqrB-T236Y mutant from Vibrio cholerae
Biological Source:
Source Organism(s):
Vibrio cholerae O395 (Taxon ID: 345073)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
3.80 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE