Deposition Date
2025-08-01
Release Date
2025-10-15
Last Version Date
2025-10-15
Entry Detail
PDB ID:
9S6I
Keywords:
Title:
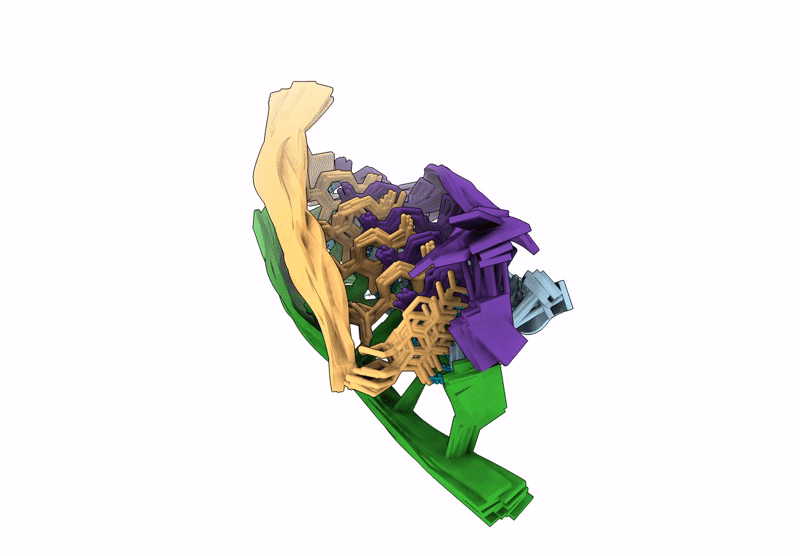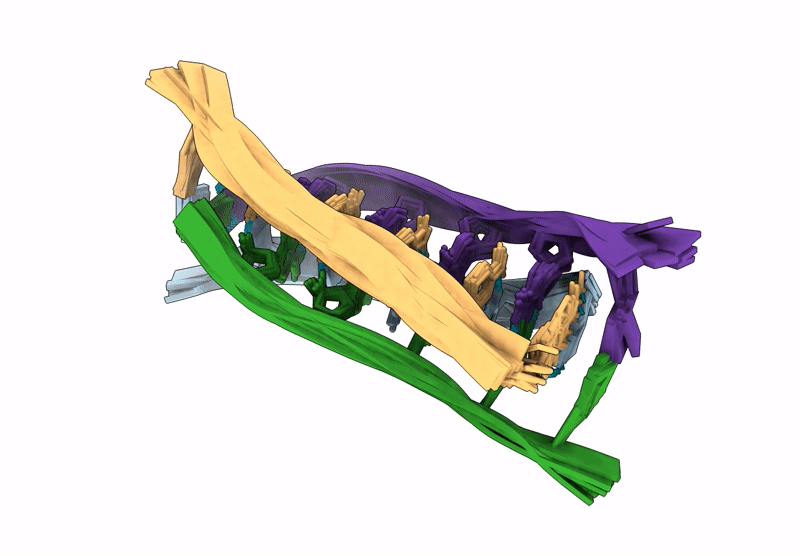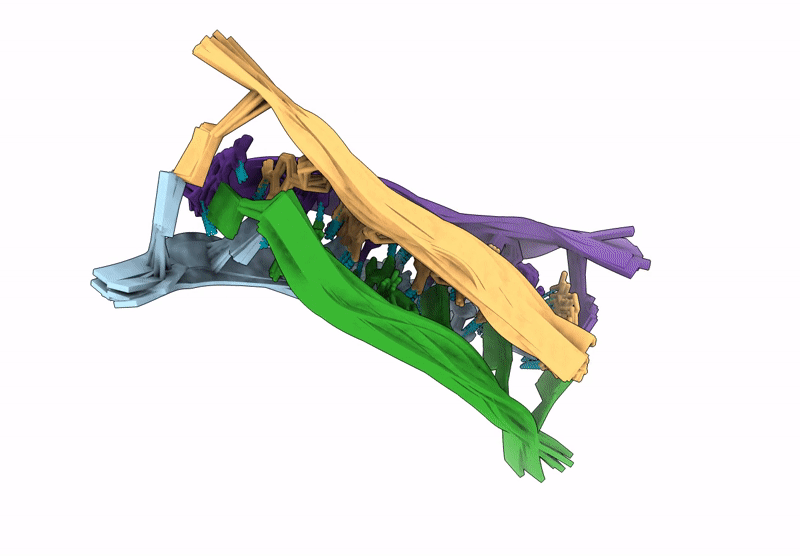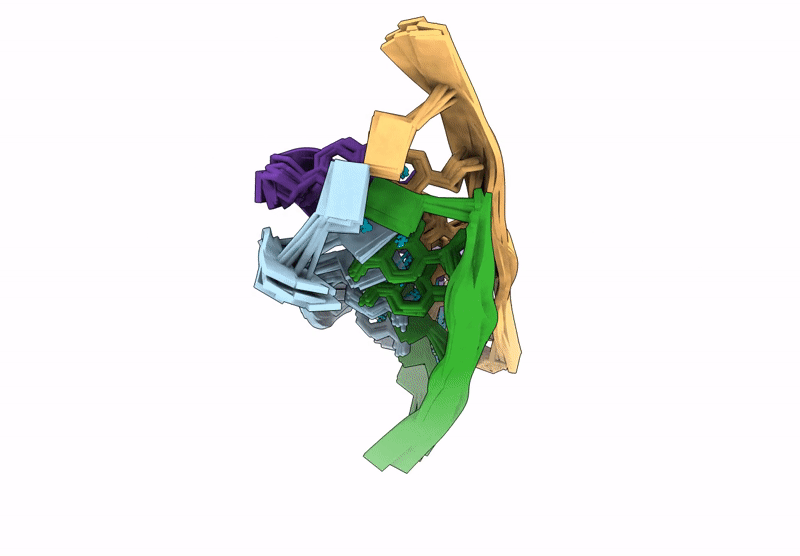
A tetrameric i-motif structure formed by dTdCdCfrCfrCdC
Biological Source:
Source Organism(s):
synthetic construct (Taxon ID: 32630)
Method Details:
Experimental Method:
Conformers Calculated:
10
Conformers Submitted:
10
Selection Criteria:
all calculated structures submitted