Deposition Date
2025-07-16
Release Date
2026-01-28
Last Version Date
2026-02-18
Entry Detail
PDB ID:
9S0E
Keywords:
Title:
3 us Intermediate-state of CBD of TtCarH at 30 mJ/cm2 laser fluence (TR-SFX, SACLA), refined against extrapolated structure factors
Biological Source:
Source Organism(s):
Thermus thermophilus (Taxon ID: 274)
Expression System(s):
Method Details:
Experimental Method:
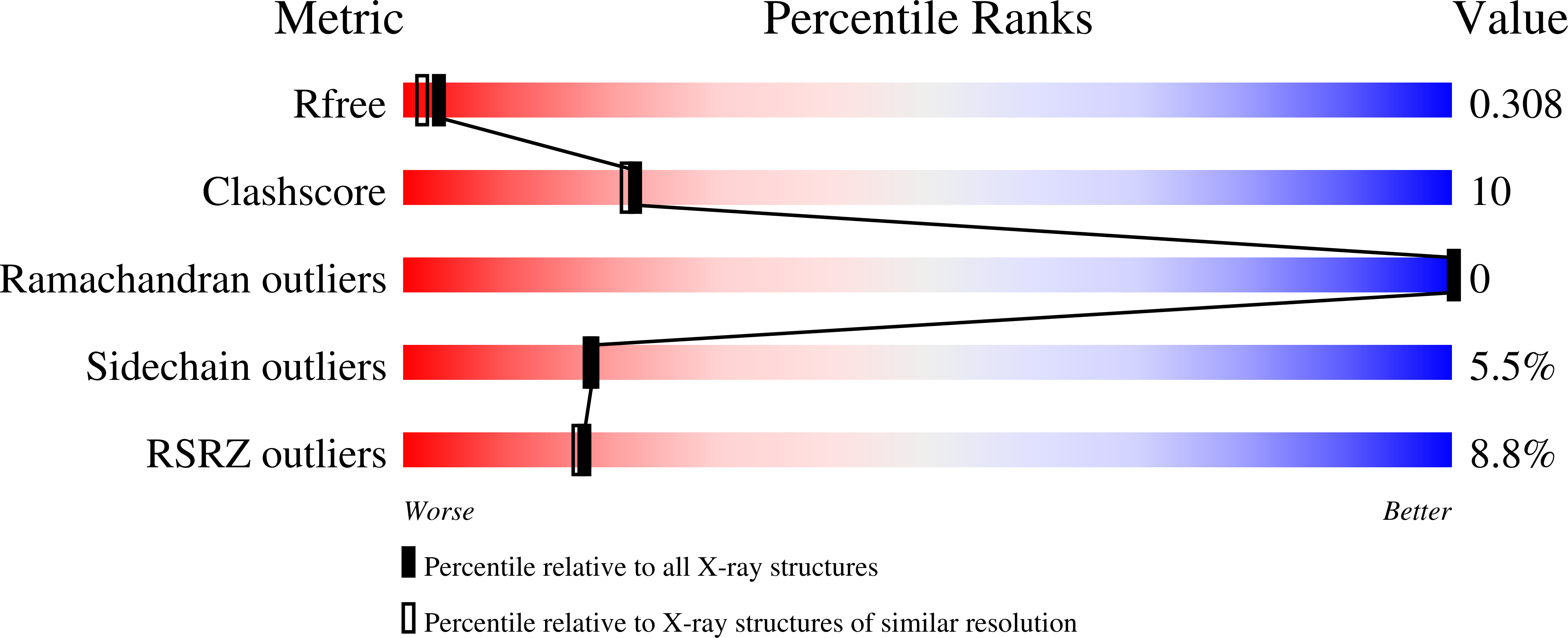
Resolution:
2.25 Å
R-Value Free:
0.30
R-Value Work:
0.26
R-Value Observed:
0.26
Space Group:
P 21 21 21