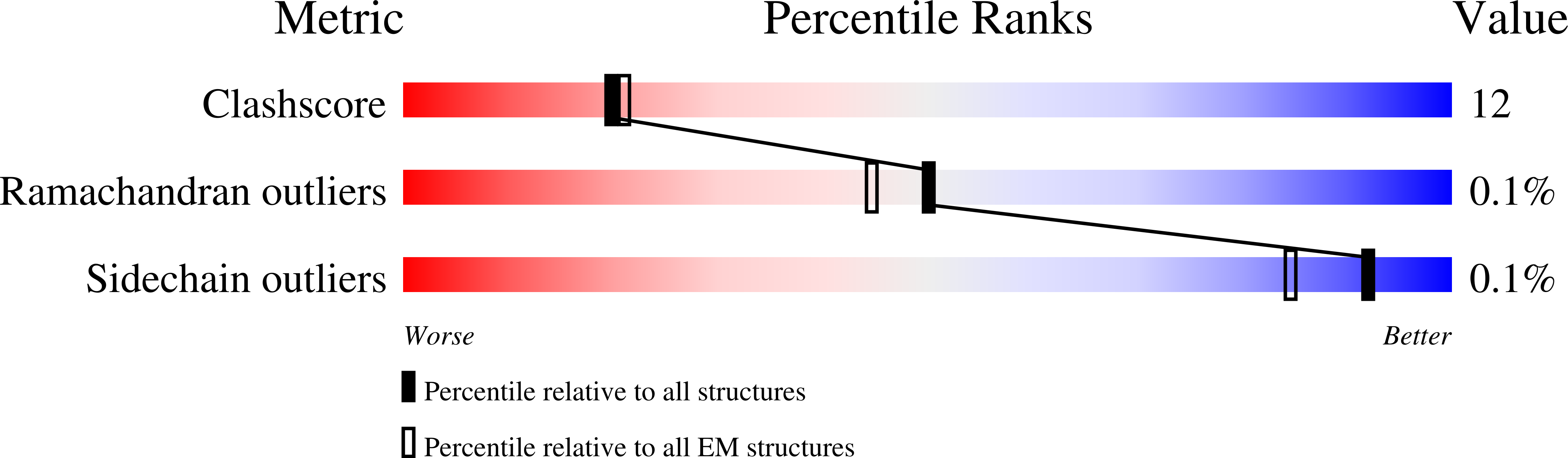
Deposition Date
2025-06-02
Release Date
2025-09-03
Last Version Date
2025-11-12
Entry Detail
PDB ID:
9RDH
Keywords:
Title:
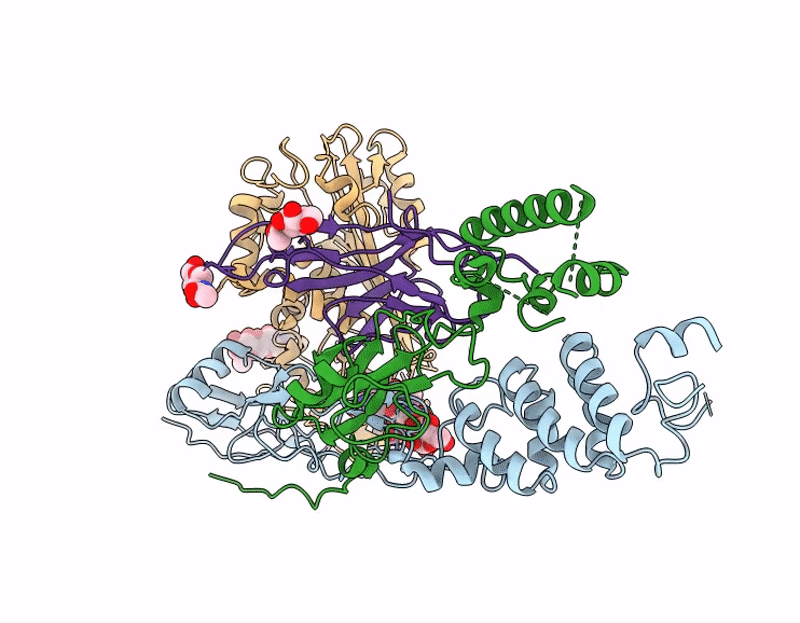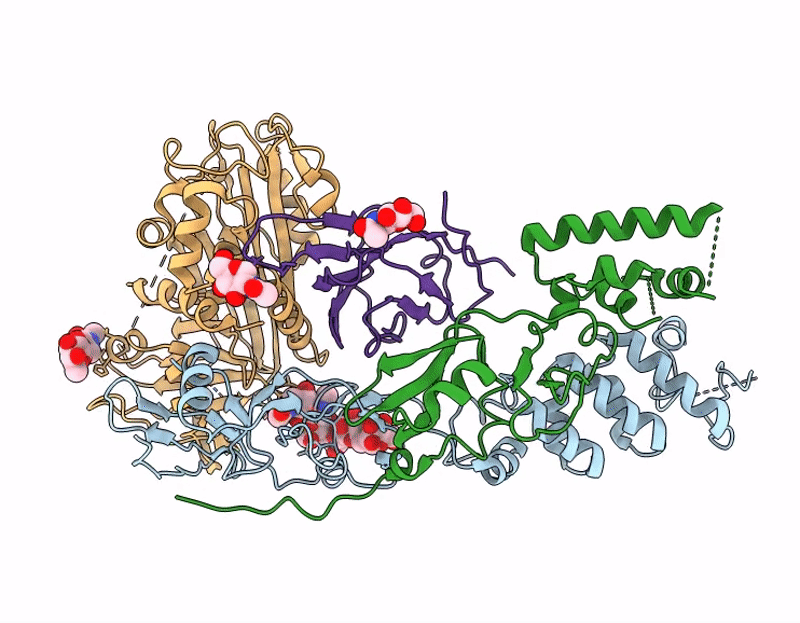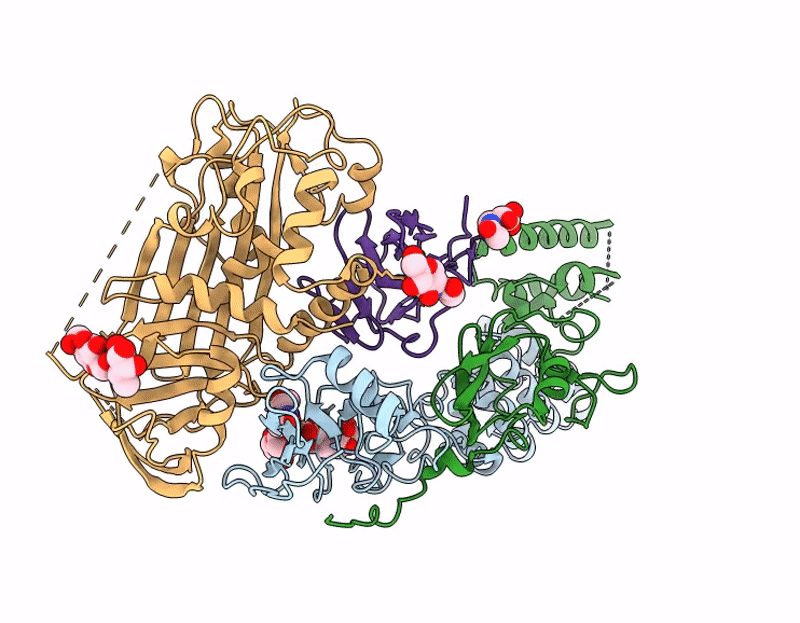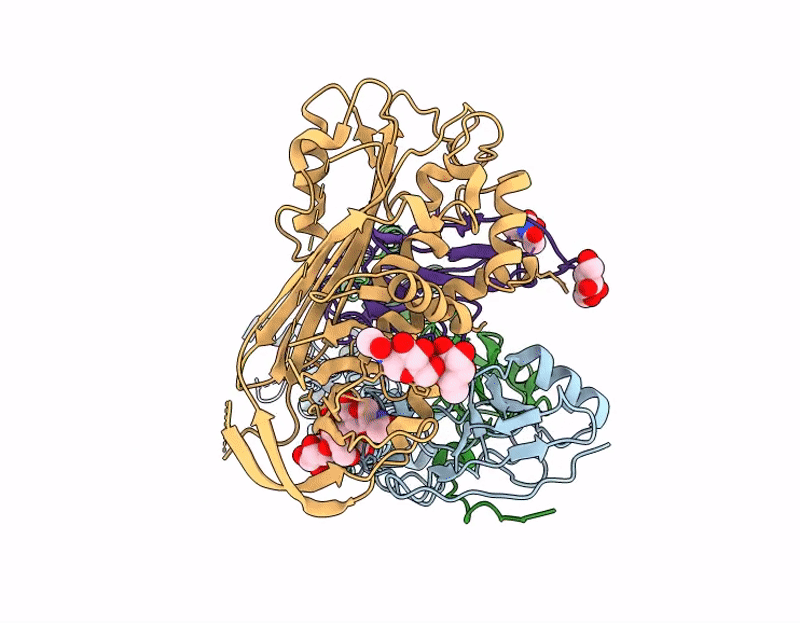
Structure of A16/G9 in complex with A56/K2 at pH 5.5 (vaccinia virus)
Biological Source:
Source Organism:
Vaccinia virus Western Reserve (Taxon ID: 696871)
Host Organism:
Method Details:
Experimental Method:
Resolution:
4.00 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE