Deposition Date
2025-05-16
Release Date
2025-07-02
Last Version Date
2026-01-14
Entry Detail
PDB ID:
9R8S
Keywords:
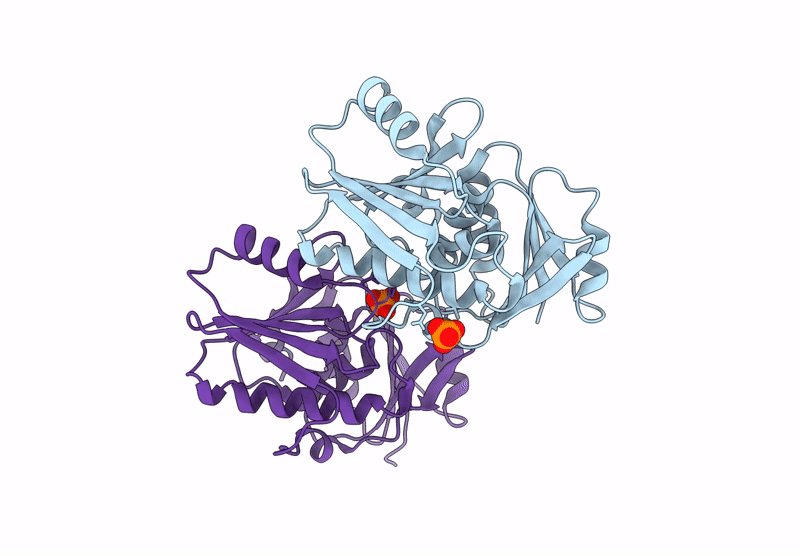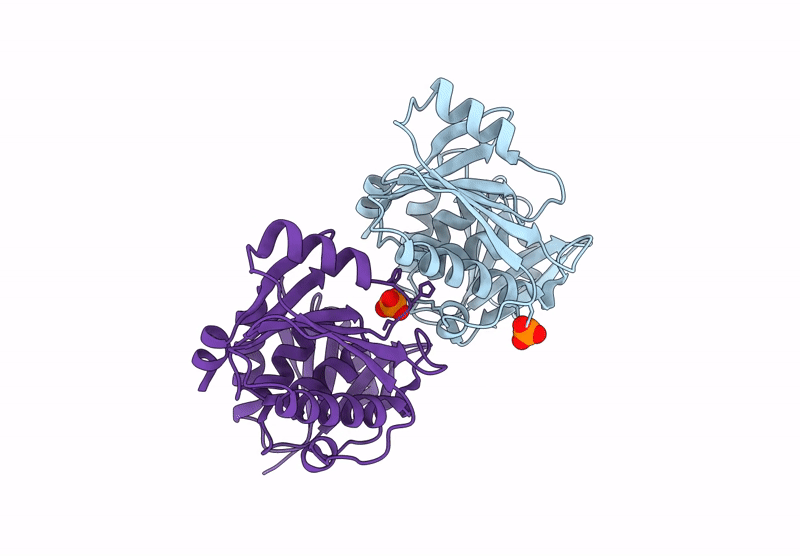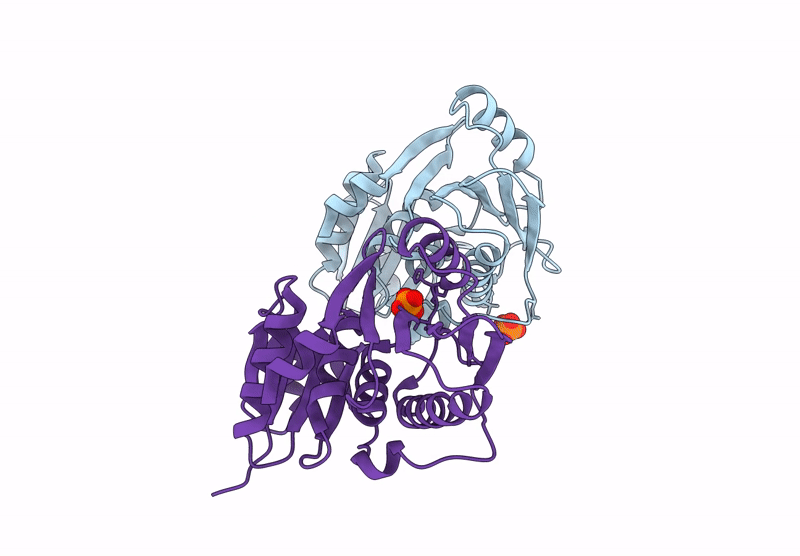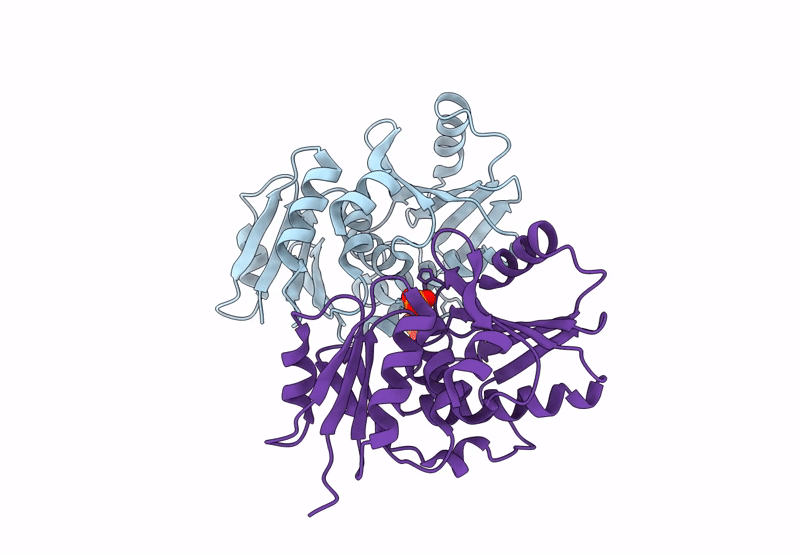
Title:
A viral SAVED protein with ring nuclease activity subverts type III CRISPR defence
Biological Source:
Source Organism(s):
Thermocrinis Great Boiling Spring virus (Taxon ID: 2770617)
Expression System(s):
Method Details:
Experimental Method:
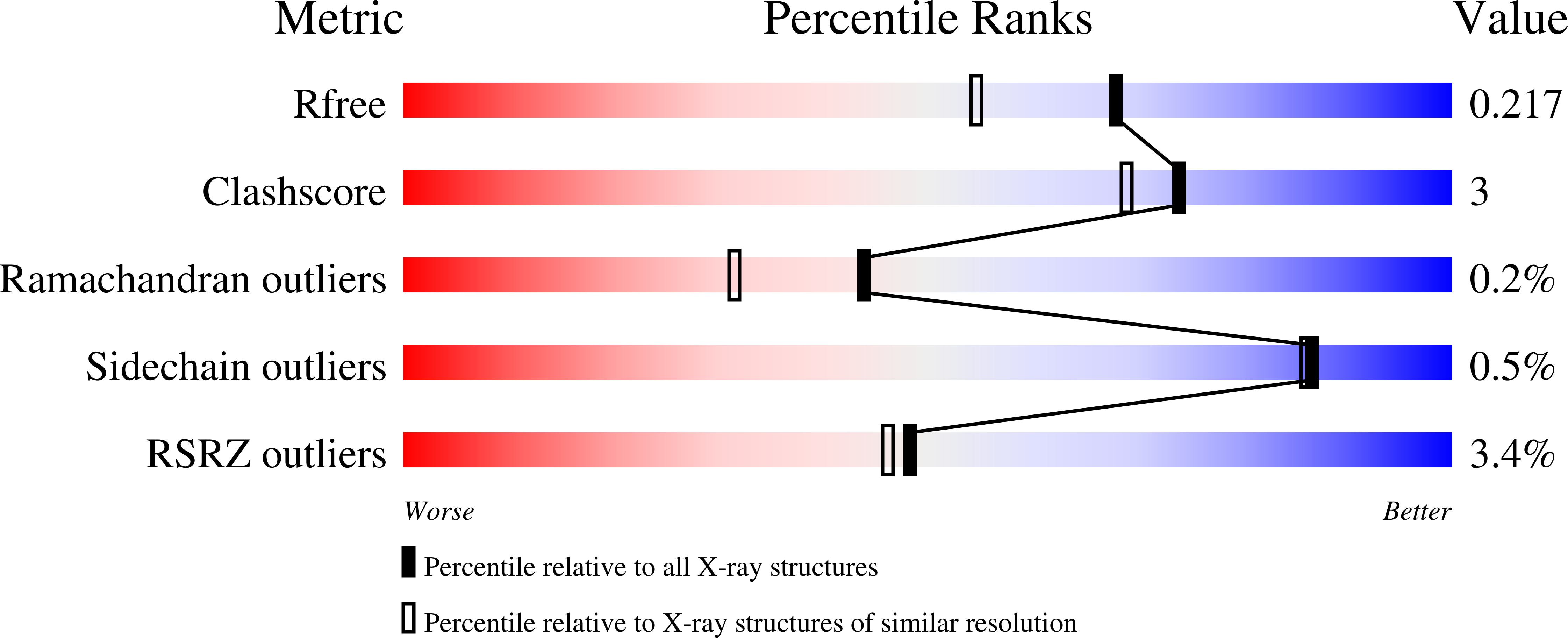
Resolution:
1.79 Å
R-Value Free:
0.20
R-Value Work:
0.16
Space Group:
P 31 2 1