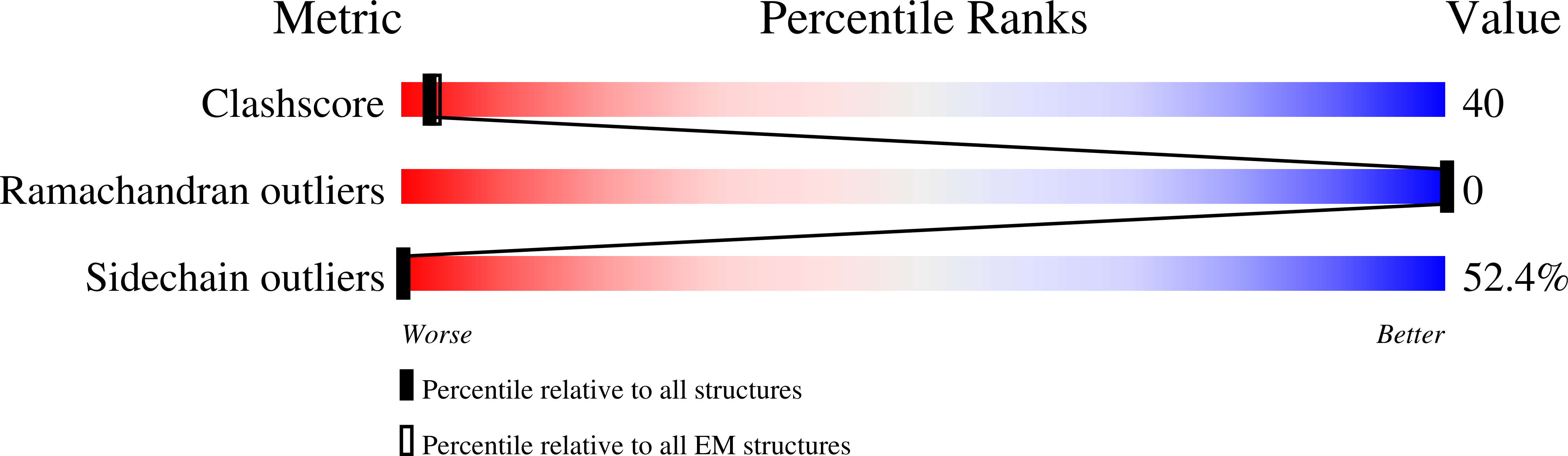
Deposition Date
2025-05-14
Release Date
2025-06-11
Last Version Date
2025-06-11
Entry Detail
PDB ID:
9R7E
EMDB ID:
Keywords:
Title:
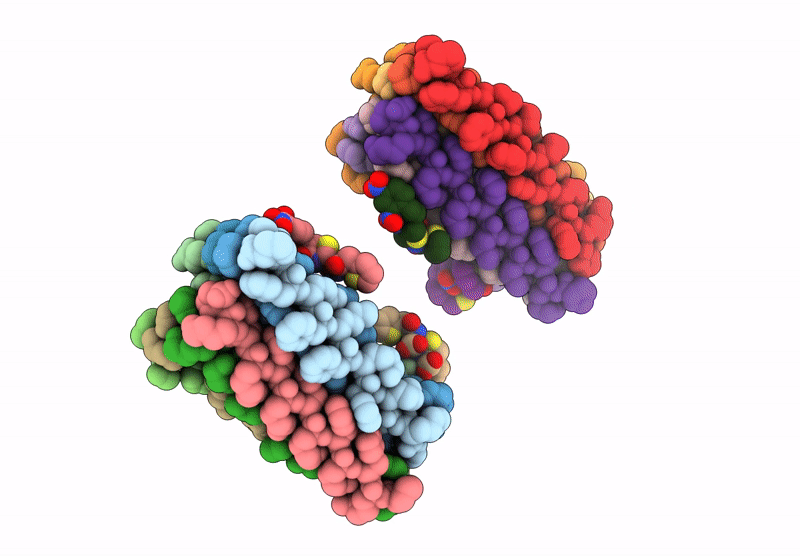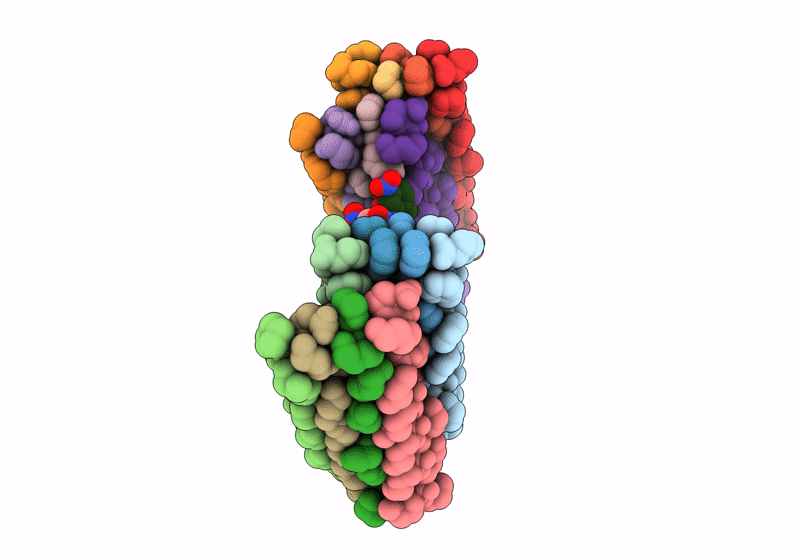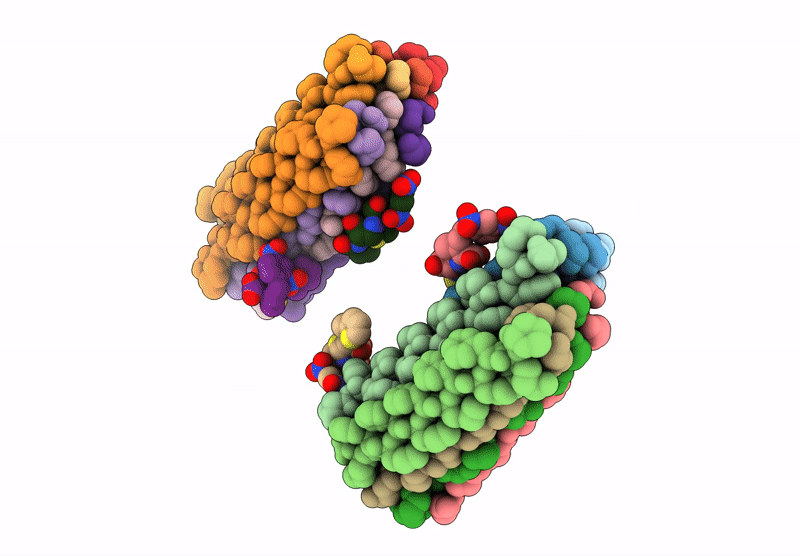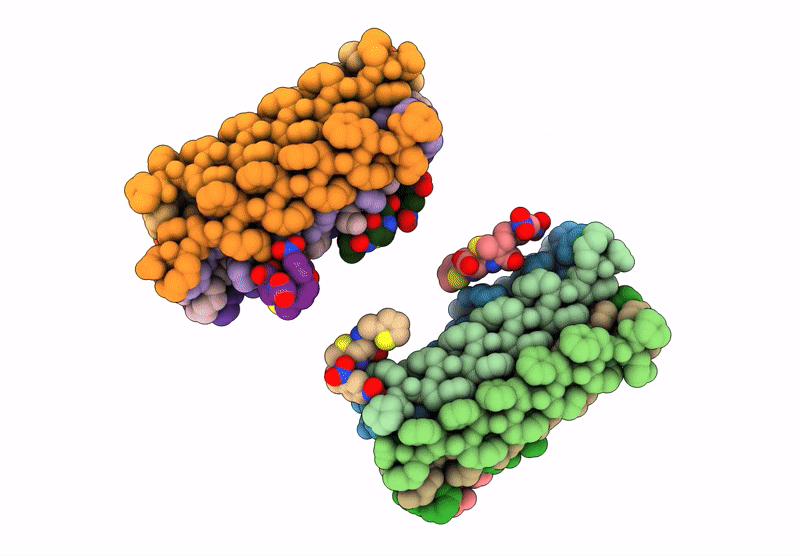
Cryo-EM Structure of catalytic amyloids
Biological Source:
Source Organism(s):
Anaeramoeba ignava (Taxon ID: 1746090)
Method Details:
Experimental Method:
Resolution:
5.00 Å
Aggregation State:
FILAMENT
Reconstruction Method:
HELICAL