Deposition Date
2025-04-04
Release Date
2025-12-03
Last Version Date
2026-01-14
Entry Detail
PDB ID:
9QS9
Keywords:
Title:
Structure and mechanism of the broad spectrum CRISPR-associated ring nuclease Crn4
Biological Source:
Source Organism(s):
Actinomyces procaprae (Taxon ID: 2560010)
synthetic construct (Taxon ID: 32630)
synthetic construct (Taxon ID: 32630)
Expression System(s):
Method Details:
Experimental Method:
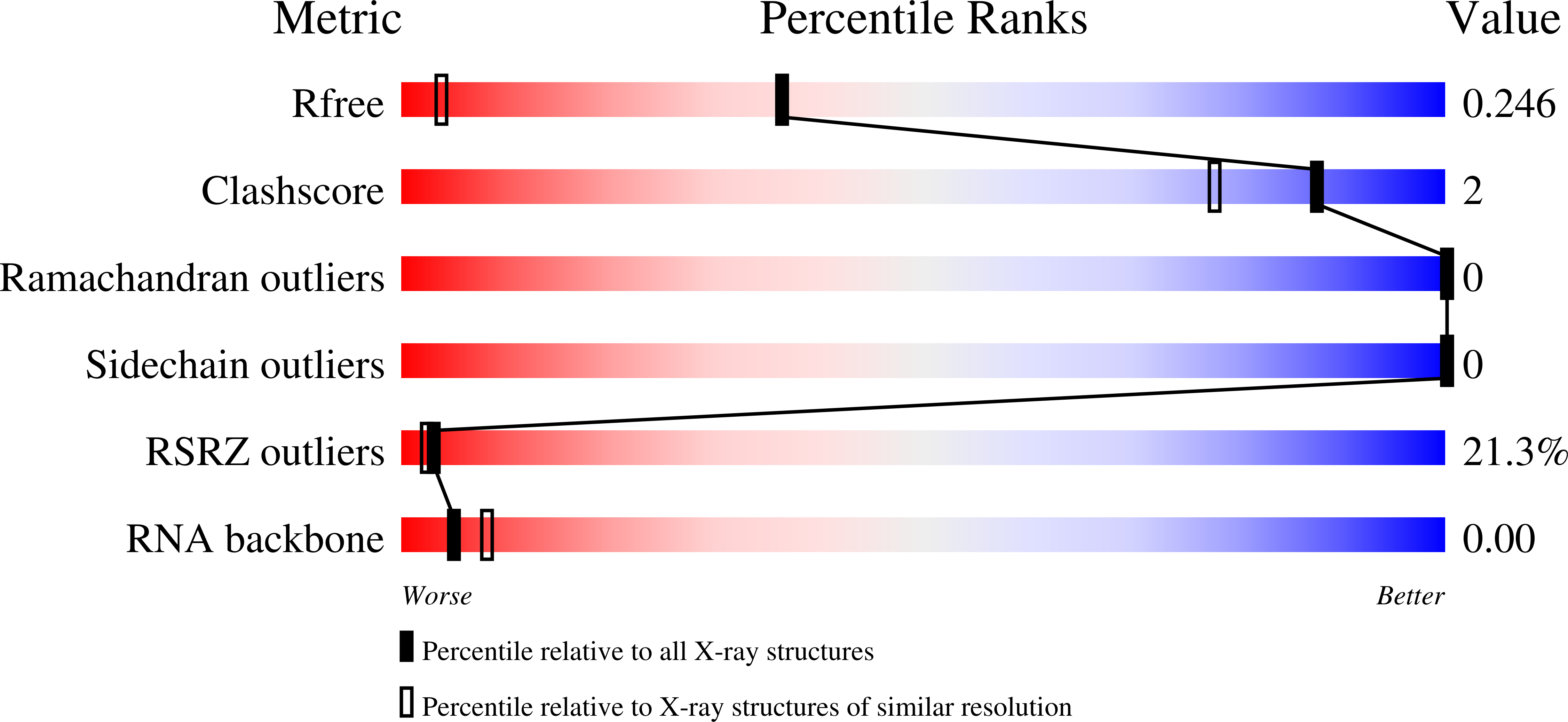
Resolution:
1.44 Å
R-Value Free:
0.23
R-Value Work:
0.22
Space Group:
P 1 21 1