Deposition Date
2025-03-21
Release Date
2025-07-16
Last Version Date
2025-09-17
Entry Detail
PDB ID:
9QM5
Keywords:
Title:
Krypton-pressurized Methyl-Coenzyme M reductase of an ANME-2c isolated from a microbial enrichment
Biological Source:
Source Organism(s):
Candidatus Methanogasteraceae archaeon (Taxon ID: 3386293)
Method Details:
Experimental Method:
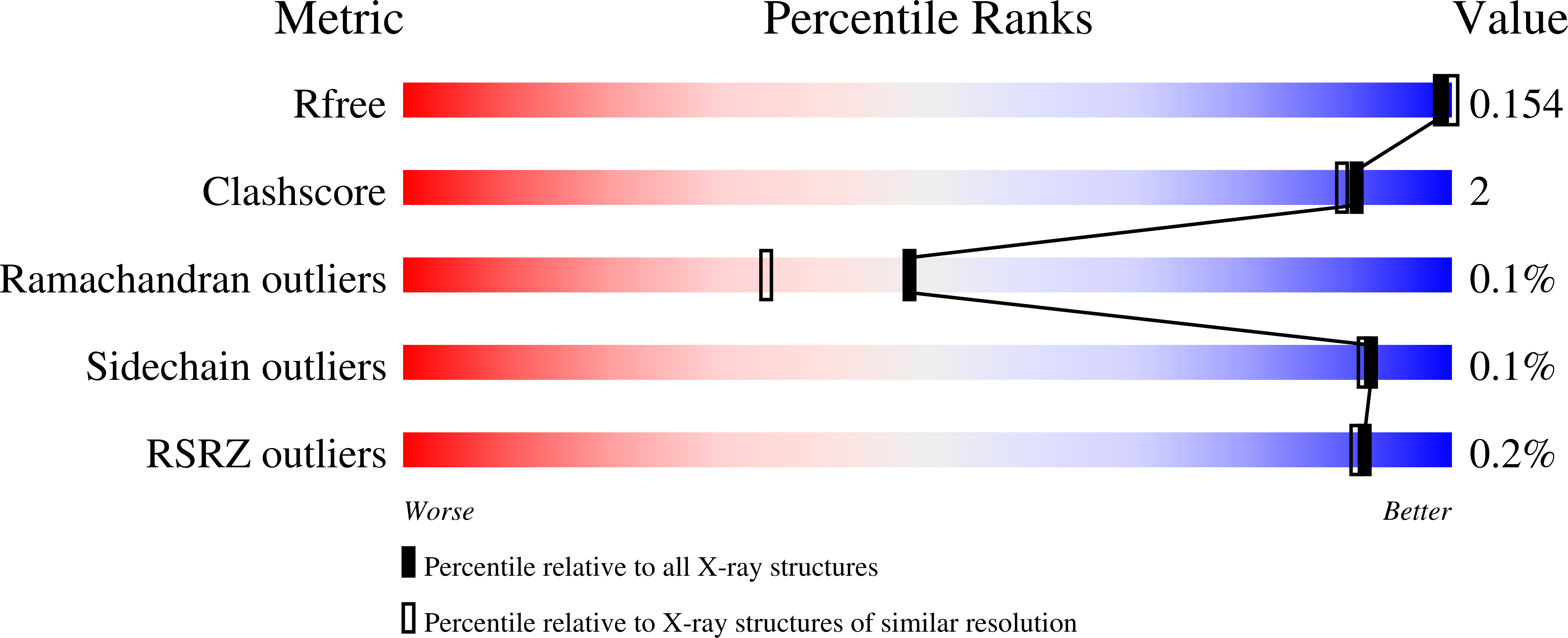
Resolution:
1.80 Å
R-Value Free:
0.15
R-Value Work:
0.12
R-Value Observed:
0.13
Space Group:
P 21 21 21