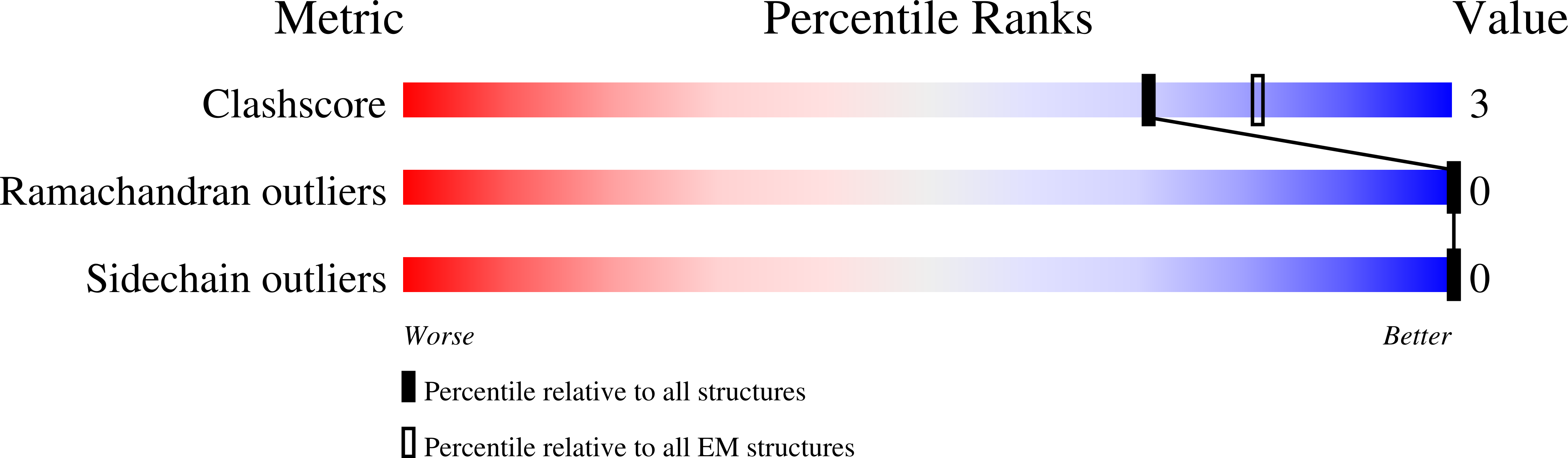
Deposition Date
2025-03-21
Release Date
2025-05-14
Last Version Date
2025-11-26
Entry Detail
PDB ID:
9QLU
Keywords:
Title:
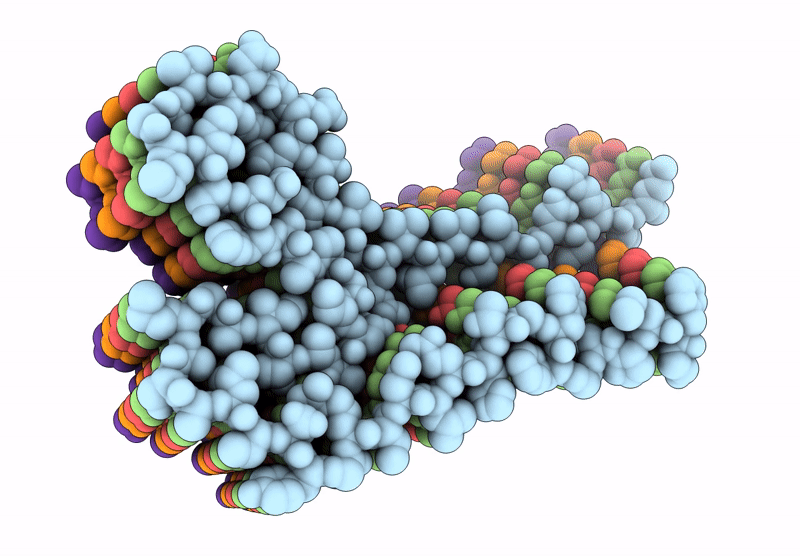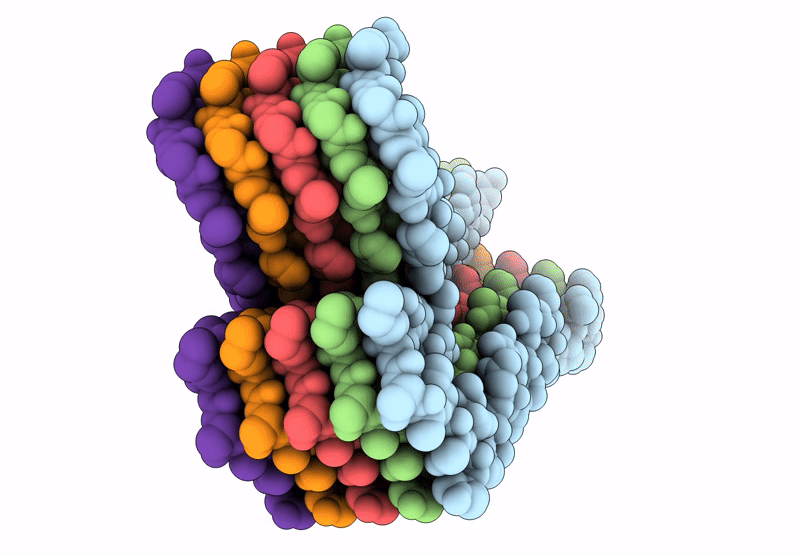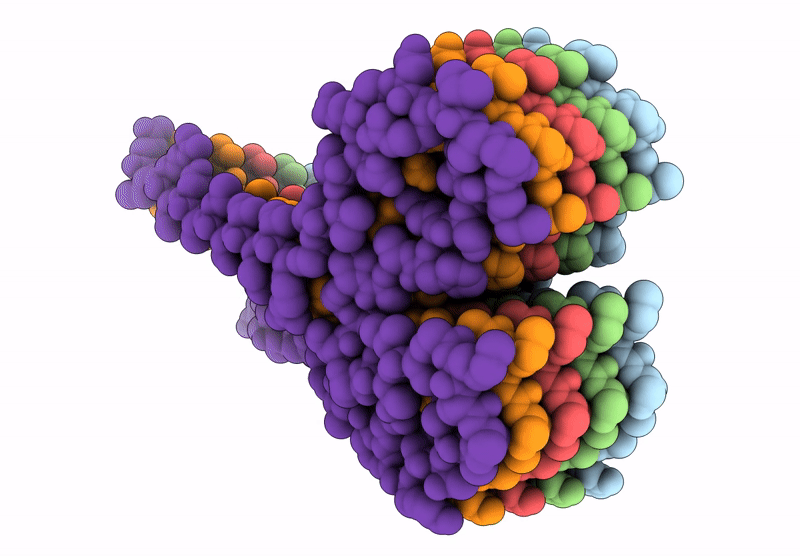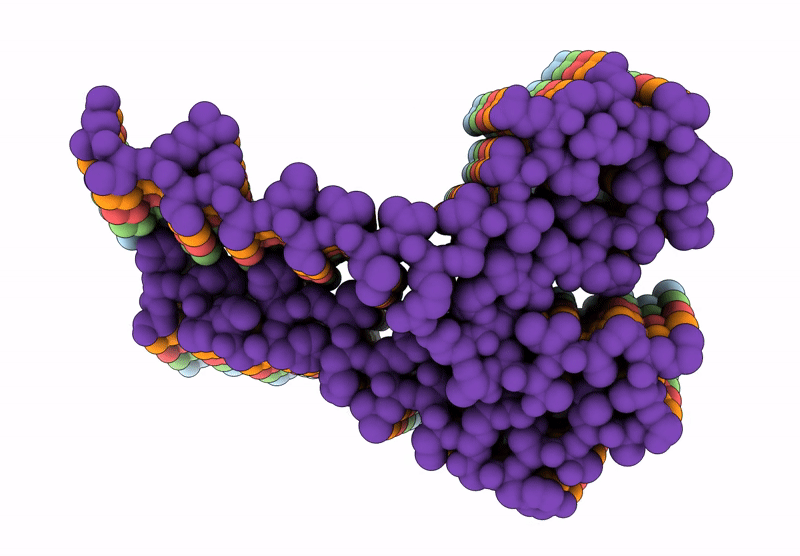
Amyloid structure of 17kDa alpha-amylase/trypsin inhibitor 2 (Uniprot ID: AI172_ORYSJ)
Biological Source:
Source Organism(s):
Oryza sativa Japonica Group (Taxon ID: 39947)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
2.54 Å
Aggregation State:
FILAMENT
Reconstruction Method:
HELICAL