Deposition Date
2025-03-06
Release Date
2025-09-10
Last Version Date
2025-12-03
Entry Detail
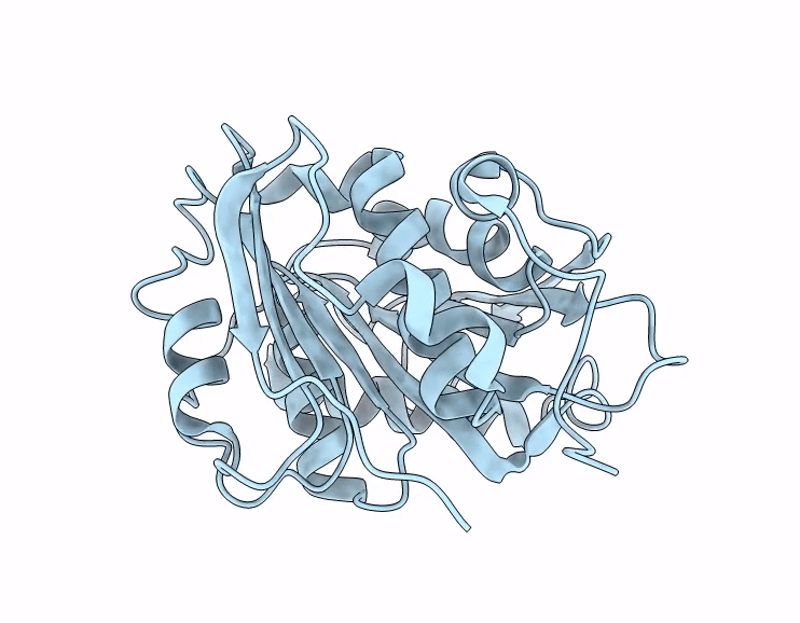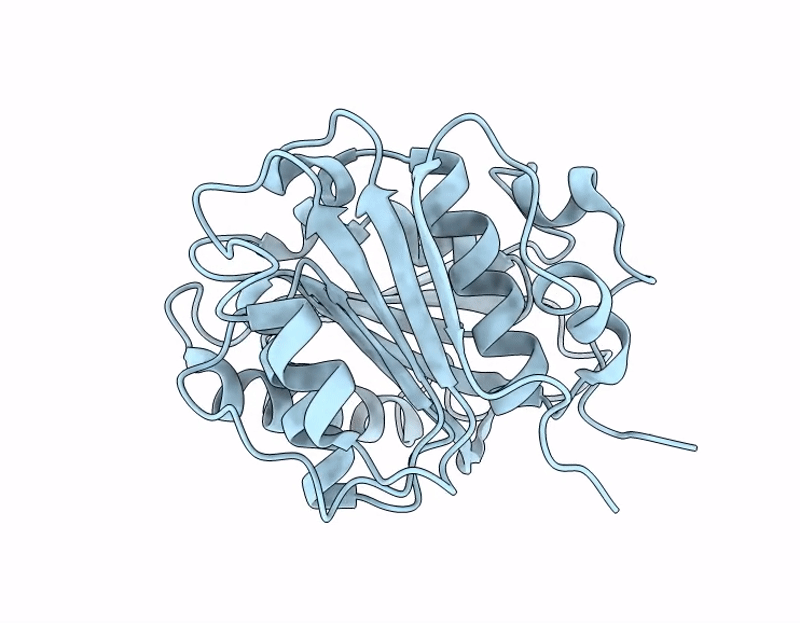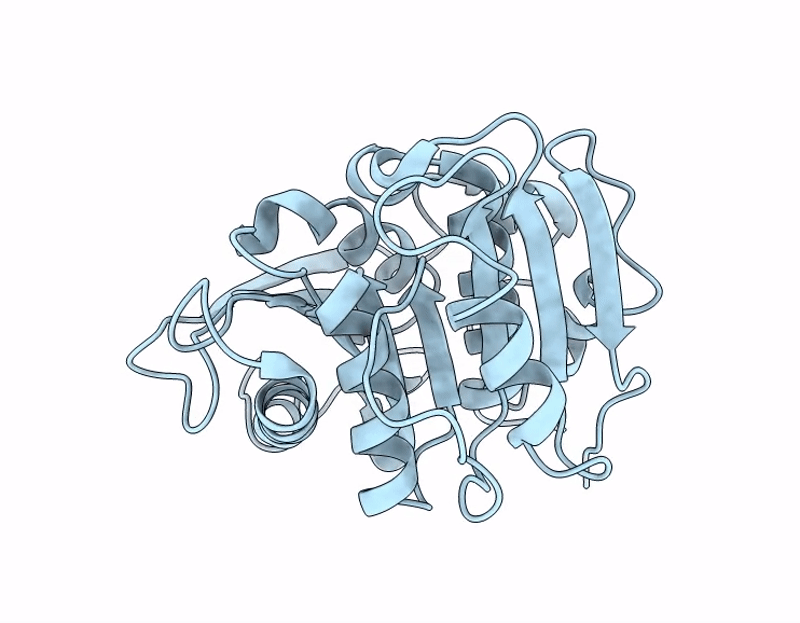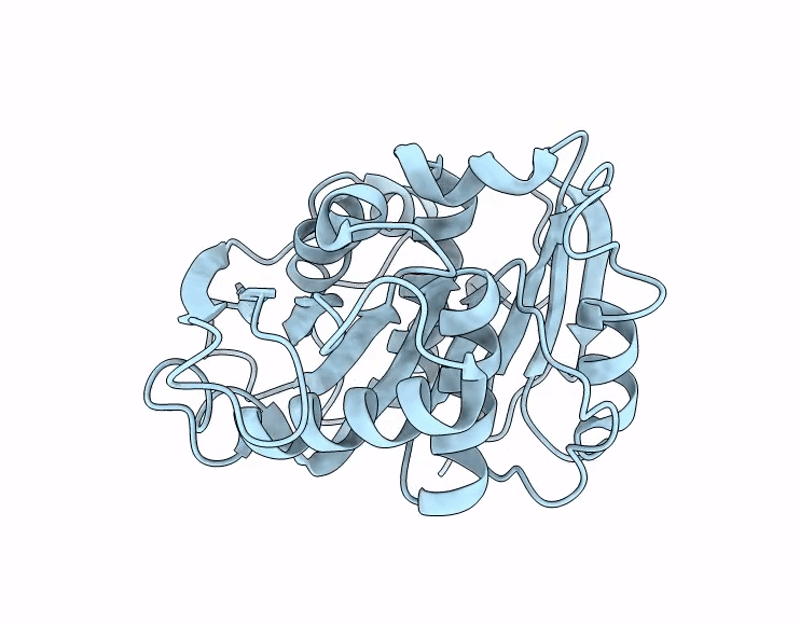
PDB ID:
9QDE
Keywords:
Title:
Crystal structure of the non-glycosylated polyester hydrolase Leipzig 7 (PHL7) mut3 variant expressed in Pichia pastoris (P_PHL7mut3_ng)
Biological Source:
Source Organism:
compost metagenome (Taxon ID: 702656)
Host Organism:
Method Details:
Experimental Method:
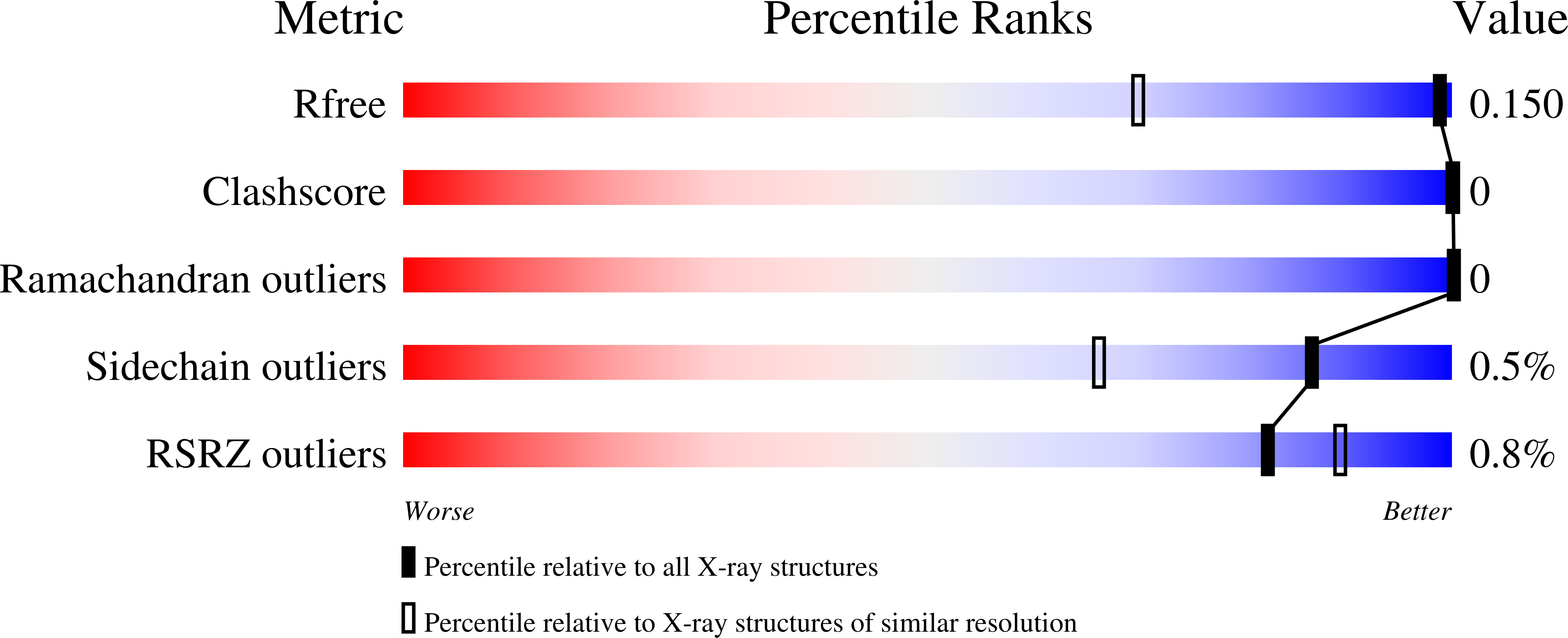
Resolution:
1.02 Å
R-Value Free:
0.14
R-Value Work:
0.12
R-Value Observed:
0.12
Space Group:
P 21 21 21