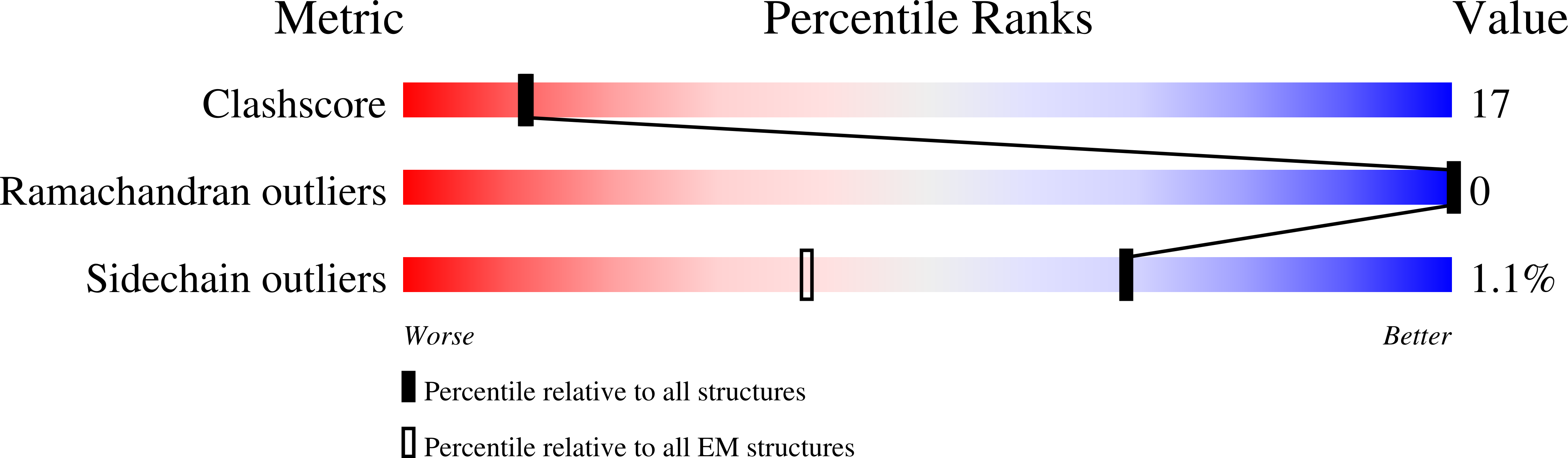Deposition Date
2025-08-18
Release Date
2025-09-24
Last Version Date
2025-09-24
Entry Detail
PDB ID:
9Q3K
Keywords:
Title:
Structure of LarA-like nickel-pincer nucleotide cofactor-utilizing enzyme with a single catalytic histidine residue from Streptococcus plurextorum
Biological Source:
Source Organism(s):
Streptococcus plurextorum (Taxon ID: 456876)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
2.20 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE