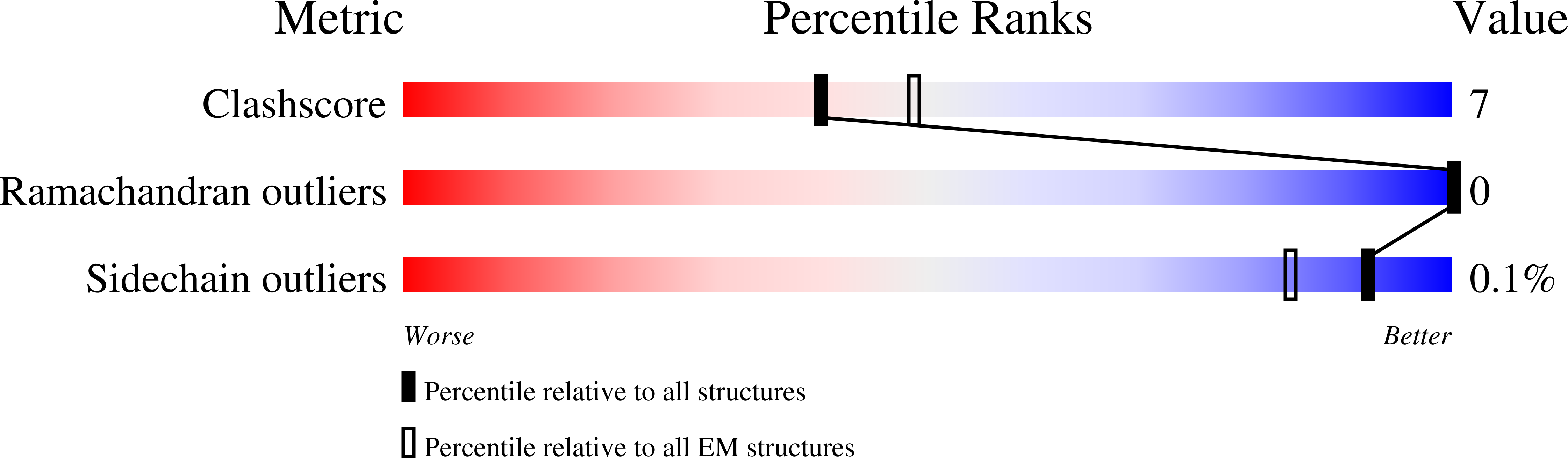Deposition Date
2025-08-18
Release Date
2025-09-17
Last Version Date
2025-09-17
Entry Detail
PDB ID:
9Q3J
Keywords:
Title:
Structures of LarA-like nickel-pincer nucleotide cofactor-utilizing enzyme with a single catalytic histidine residue from Blautia wexlerae
Biological Source:
Source Organism(s):
Blautia wexlerae (Taxon ID: 418240)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
3.15 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE