Deposition Date
2025-07-24
Release Date
2025-12-31
Last Version Date
2025-12-31
Entry Detail
PDB ID:
9PRU
Keywords:
Title:
Complex of the 3G8 Fab bound to Fc gamma receptor 3a / CD16a F158 allotype
Biological Source:
Source Organism:
Mus musculus (Taxon ID: 10090)
Homo sapiens (Taxon ID: 9606)
Homo sapiens (Taxon ID: 9606)
Host Organism:
Method Details:
Experimental Method:
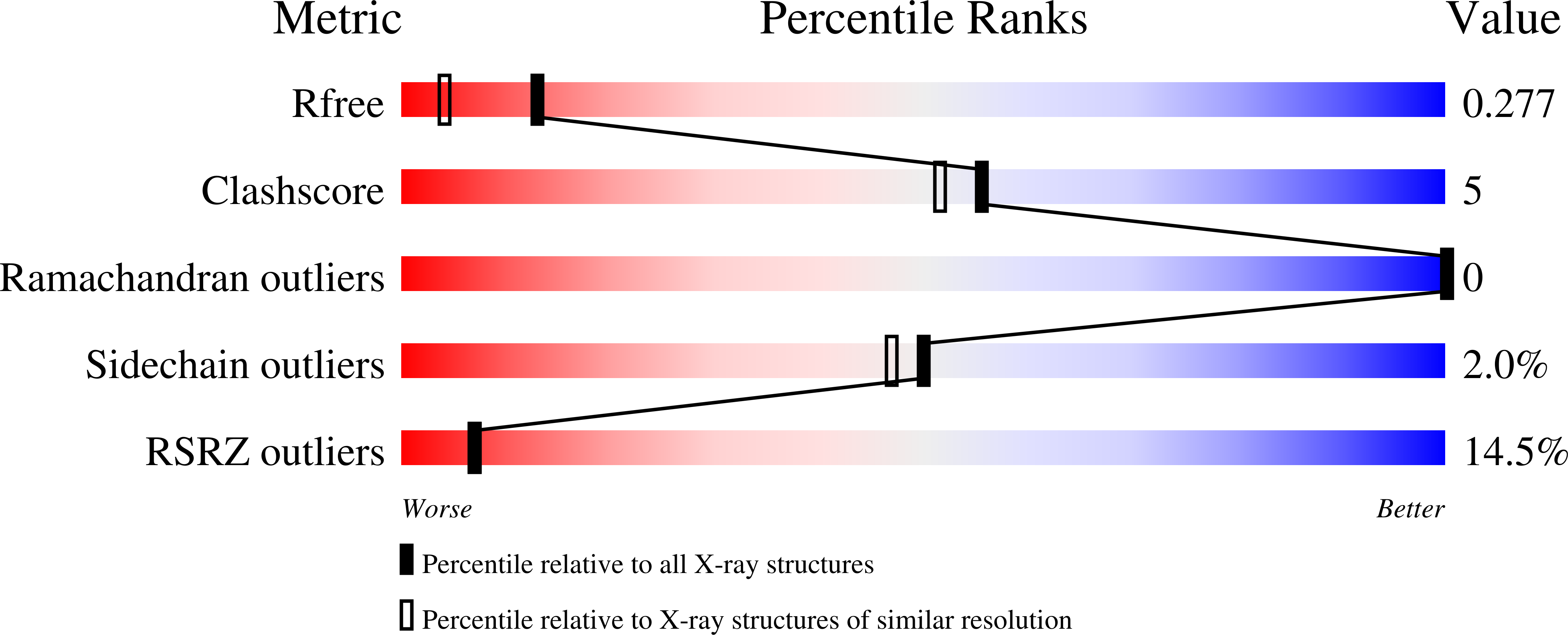
Resolution:
1.90 Å
R-Value Free:
0.28
R-Value Work:
0.23
R-Value Observed:
0.23
Space Group:
P 21 21 21