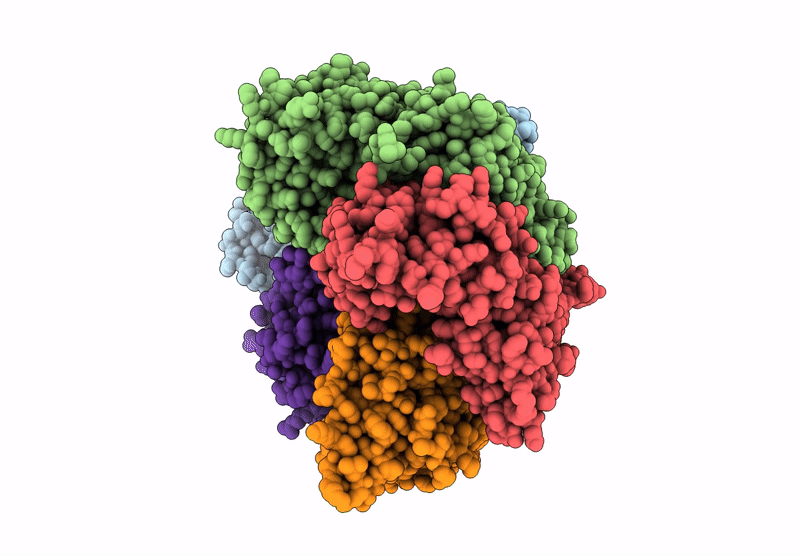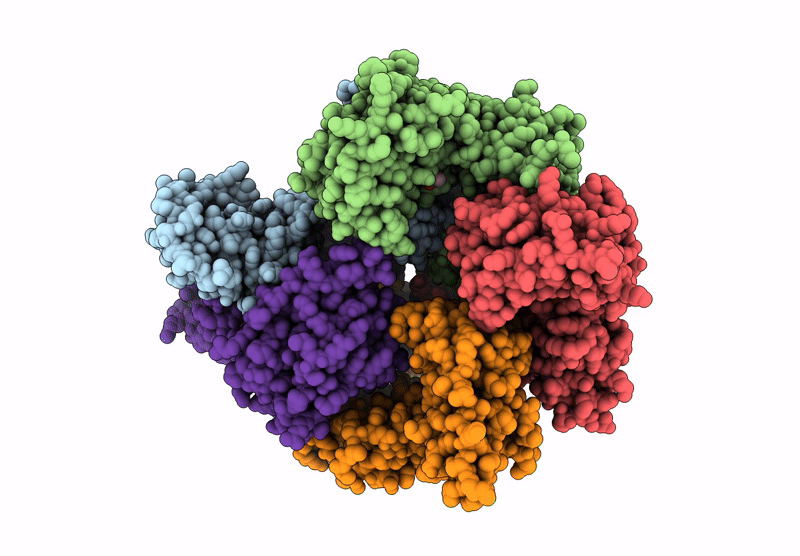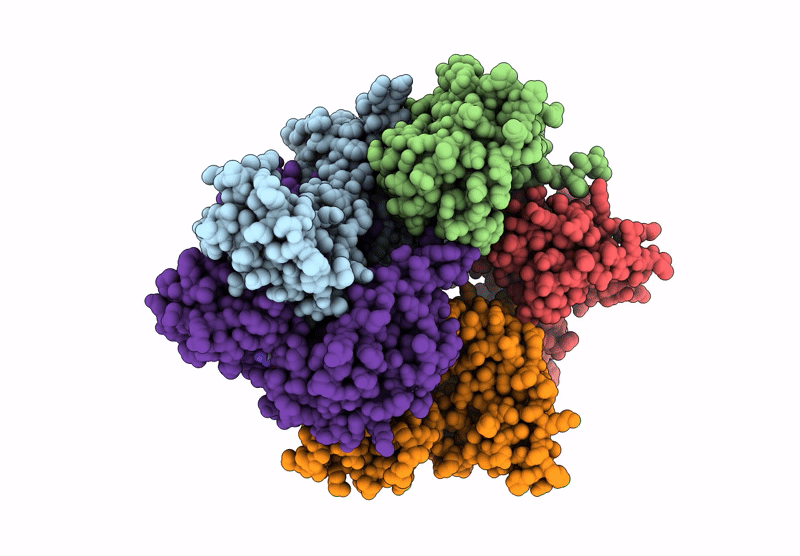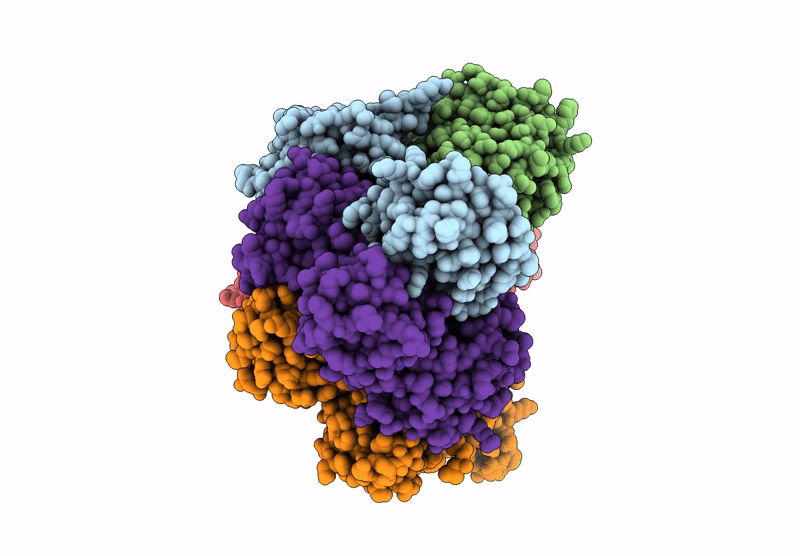
Deposition Date
2025-07-02
Release Date
2025-11-12
Last Version Date
2025-11-26
Entry Detail
PDB ID:
9PET
Keywords:
Title:
Structure of the S. cerevisiae clamp loader Replication Factor C (RFC) with mixed nucleotide occupancy
Biological Source:
Source Organism(s):
Saccharomyces cerevisiae (Taxon ID: 4932)
Expression System(s):
Method Details:
Experimental Method:
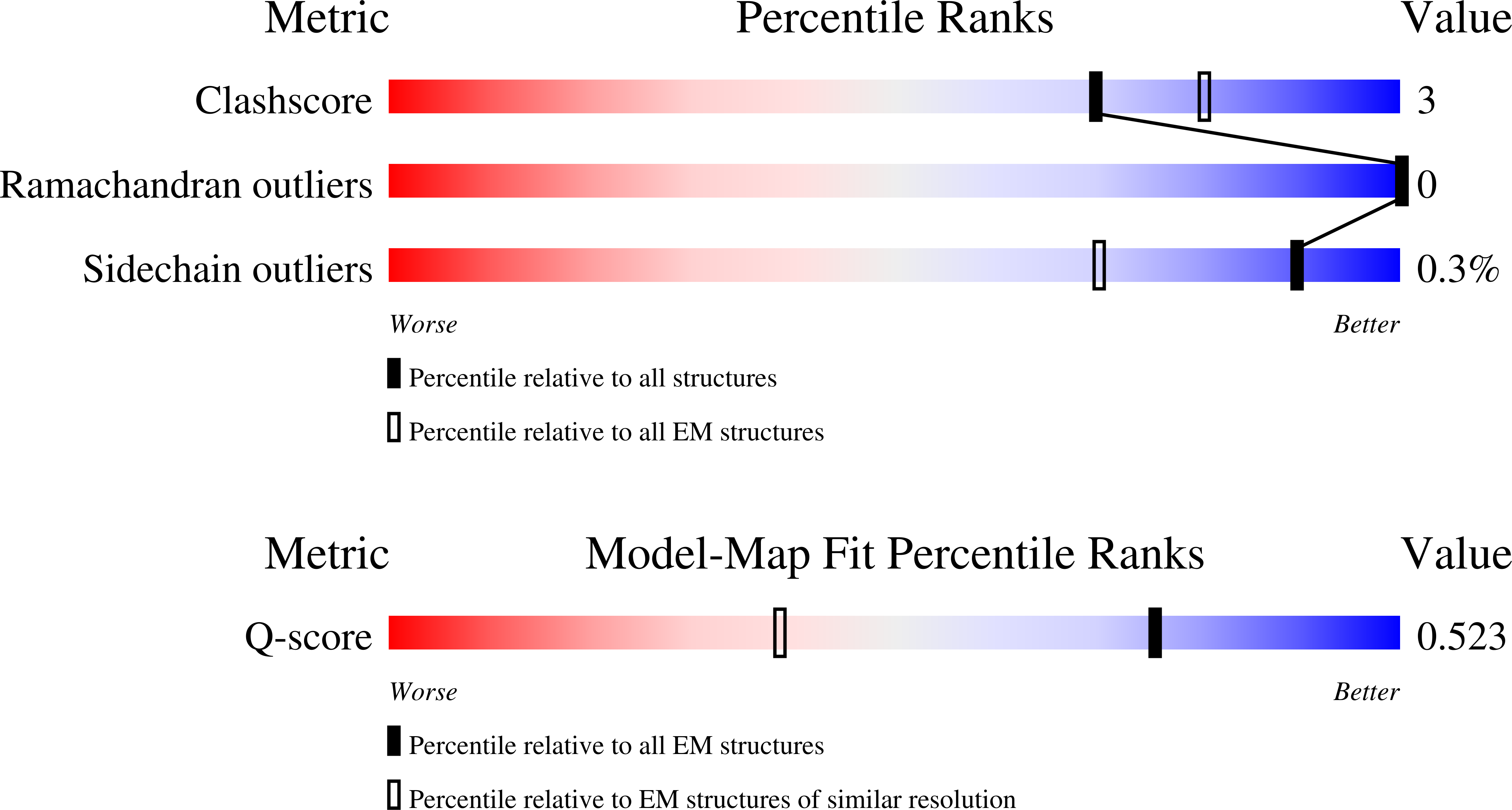
Resolution:
2.57 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE