Deposition Date
2025-05-21
Release Date
2025-10-08
Last Version Date
2025-10-08
Entry Detail
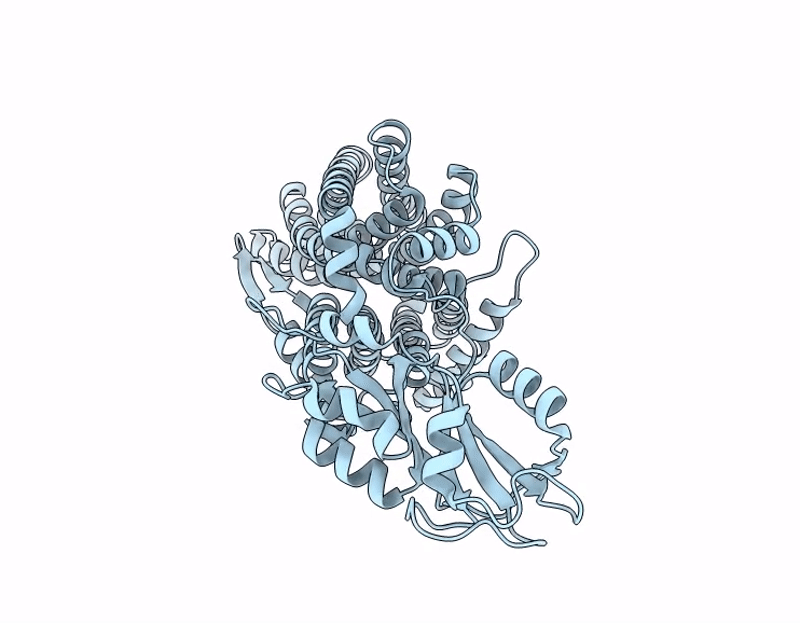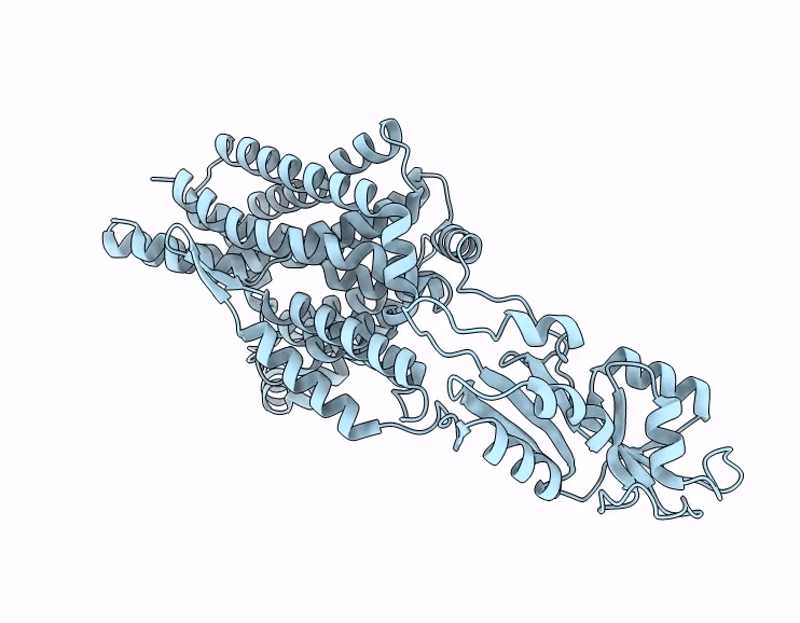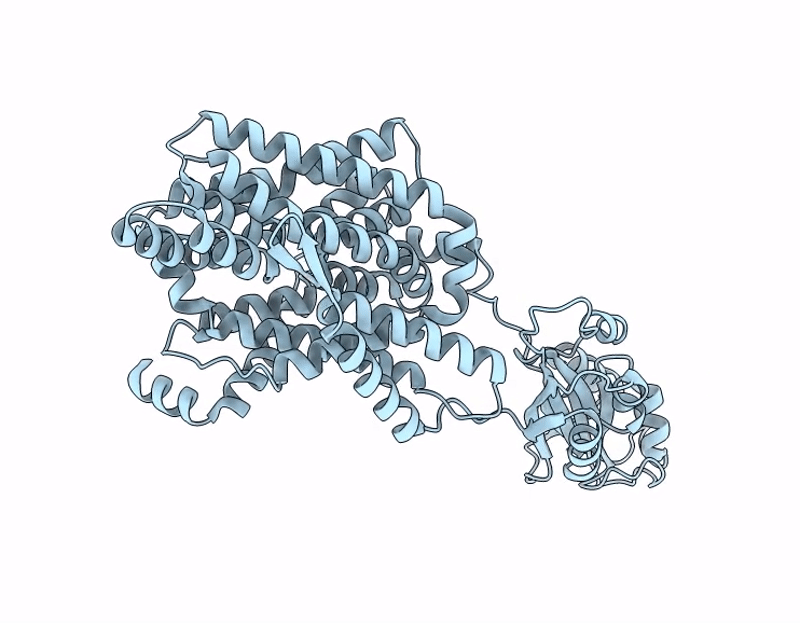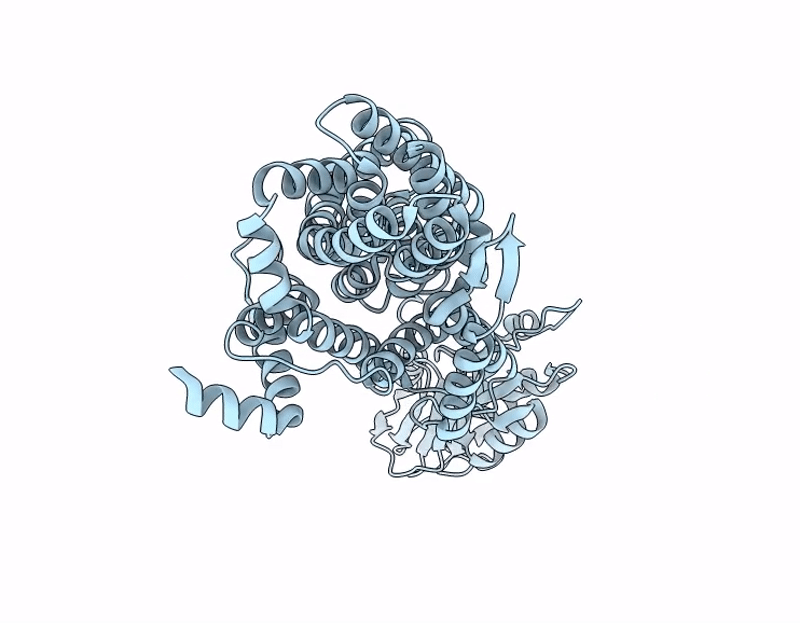
PDB ID:
9OQY
Keywords:
Title:
X-ray crystal structure of Asp/Ala exchanger AspT at outward-facing conformation
Biological Source:
Source Organism:
Tetragenococcus halophilus (Taxon ID: 51669)
Host Organism:
Method Details:
Experimental Method:
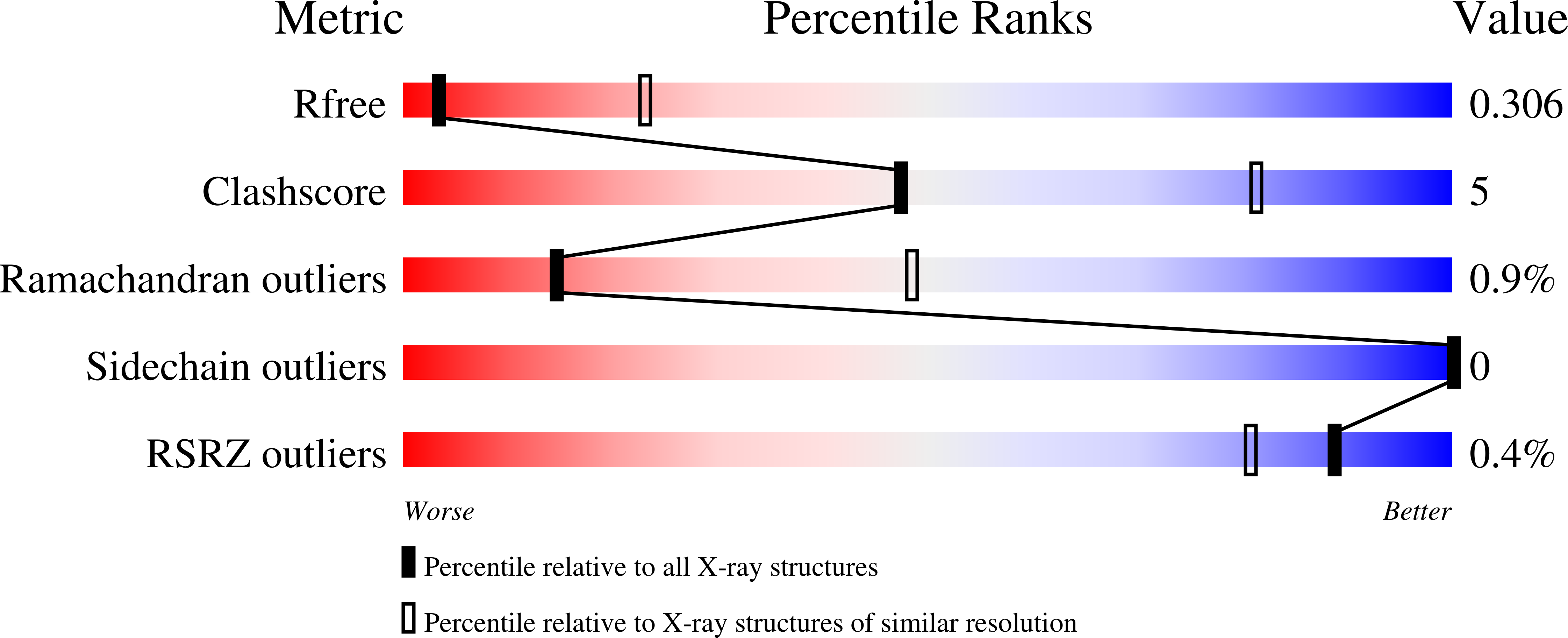
Resolution:
3.45 Å
R-Value Free:
0.30
R-Value Work:
0.25
R-Value Observed:
0.25
Space Group:
I 41 2 2