Deposition Date
2025-05-21
Release Date
2025-12-03
Last Version Date
2025-12-03
Entry Detail
PDB ID:
9OQX
Keywords:
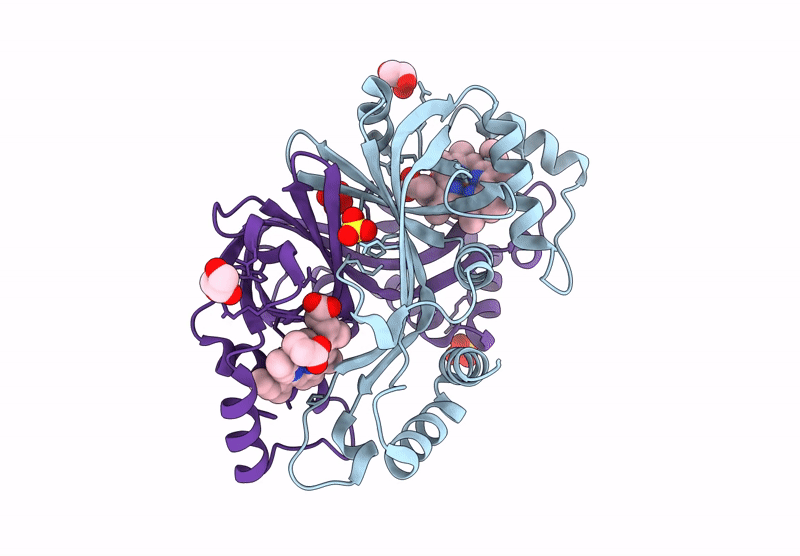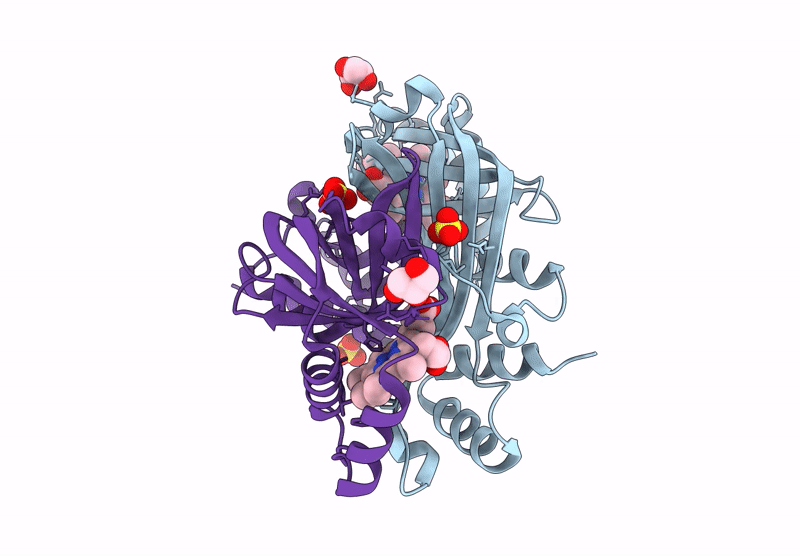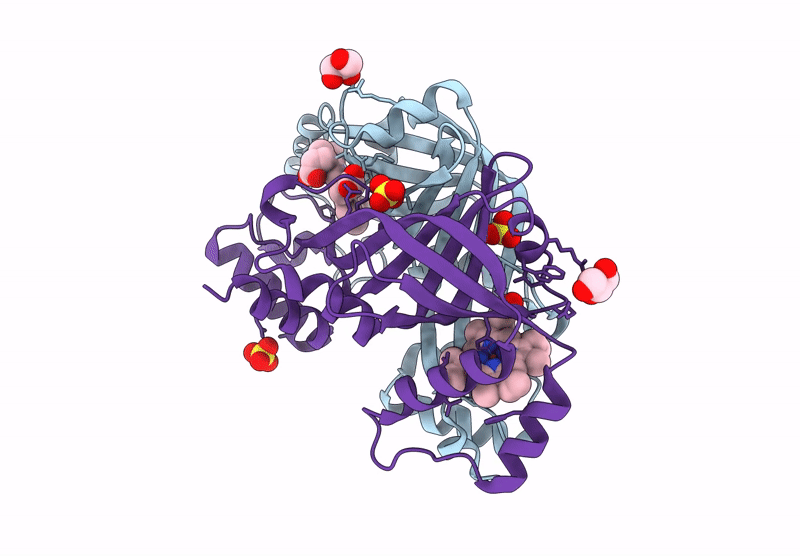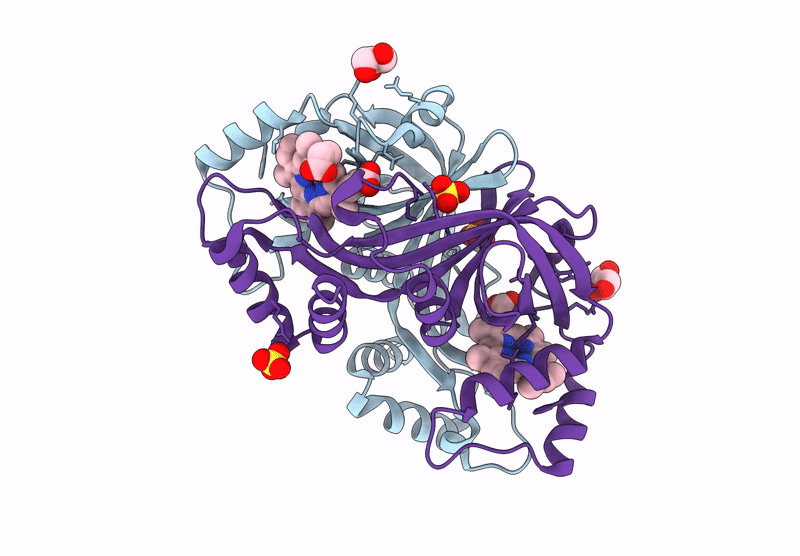
Title:
N-hydroxylamine dehydratase (NohD) H2F/F4P/P5S/R6Y/R144Y/V96A (P1/A1/A2) mutant crystal structure with heme
Biological Source:
Source Organism:
Actinomadura luzonensis (Taxon ID: 2805427)
Host Organism:
Method Details:
Experimental Method:
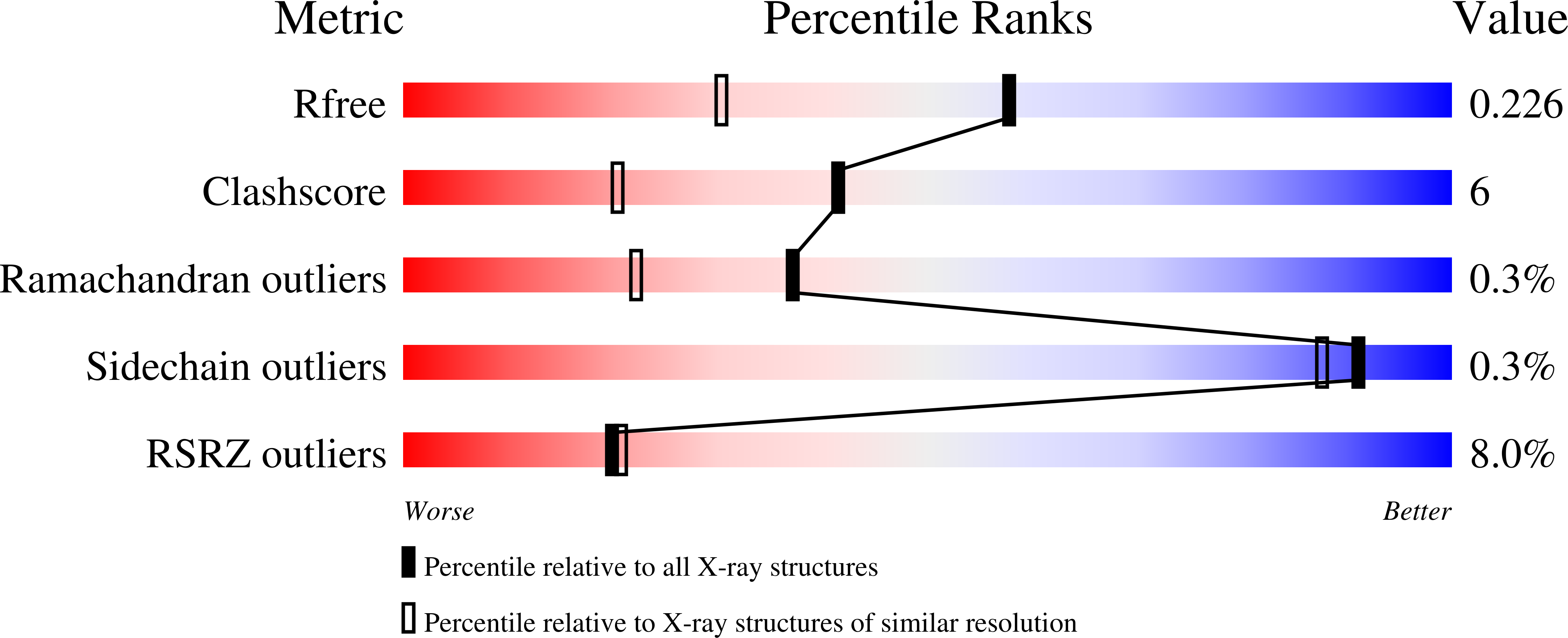
Resolution:
1.68 Å
R-Value Free:
0.22
R-Value Work:
0.19
R-Value Observed:
0.19
Space Group:
P 41 21 2