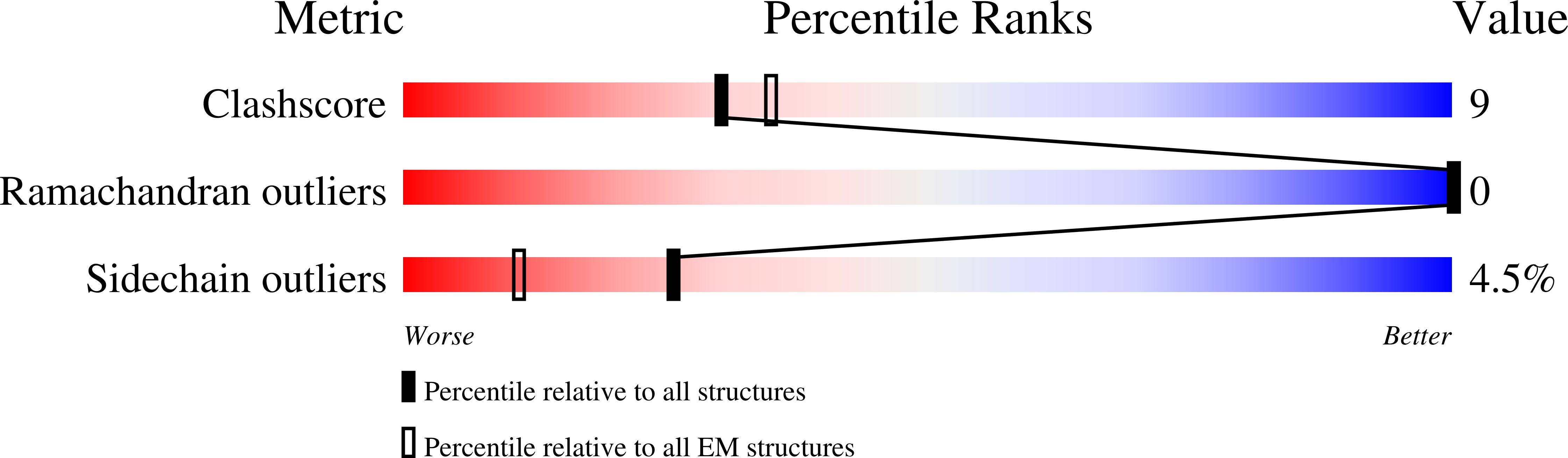
Deposition Date
2025-05-20
Release Date
2025-08-13
Last Version Date
2025-08-20
Entry Detail
PDB ID:
9OQA
Keywords:
Title:
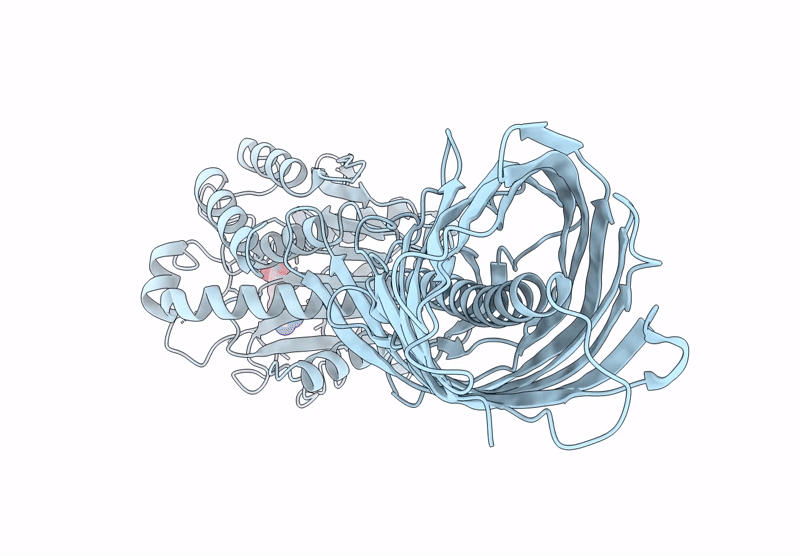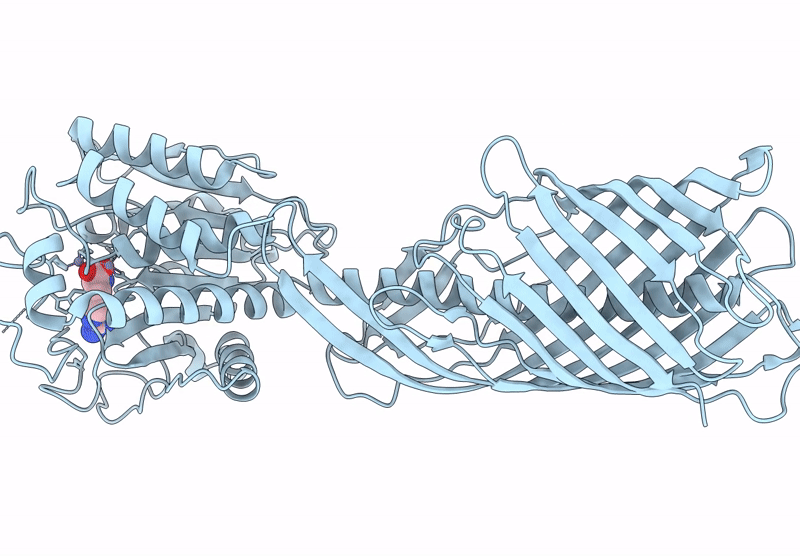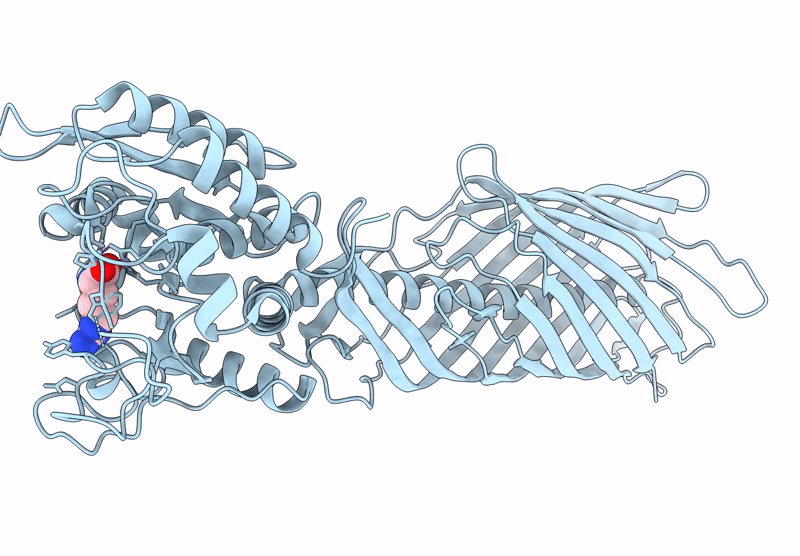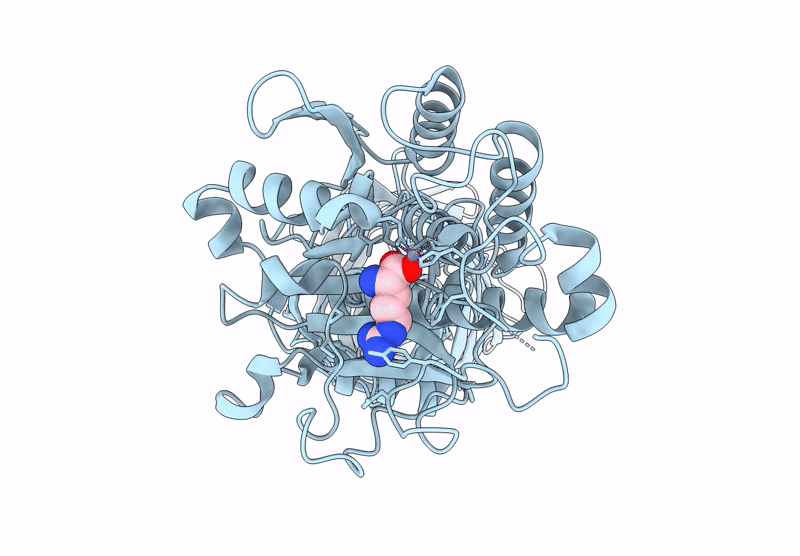
Cryo-EM structure of AaaA, a Pseudomonas Aeruginosa autotransporter
Biological Source:
Source Organism:
Pseudomonas aeruginosa (Taxon ID: 287)
Host Organism:
Method Details:
Experimental Method:
Resolution:
3.87 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE