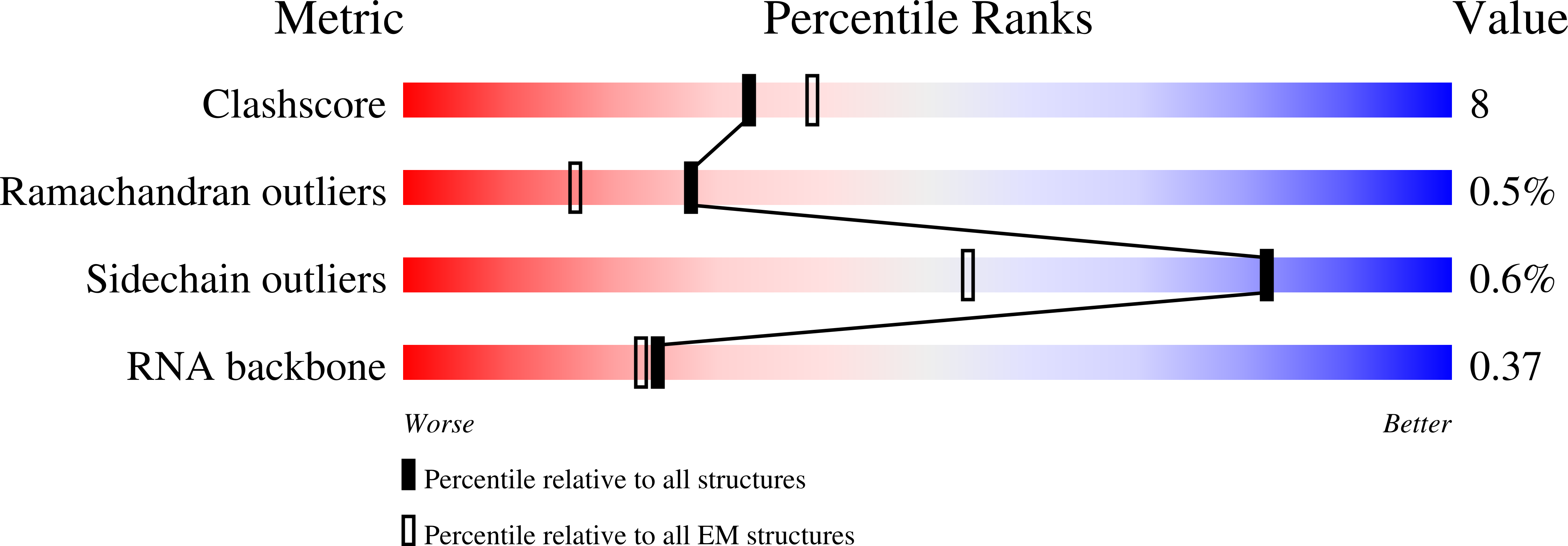
Deposition Date
2025-03-02
Release Date
2025-06-18
Last Version Date
2025-07-02
Entry Detail
PDB ID:
9NL3
Keywords:
Title:
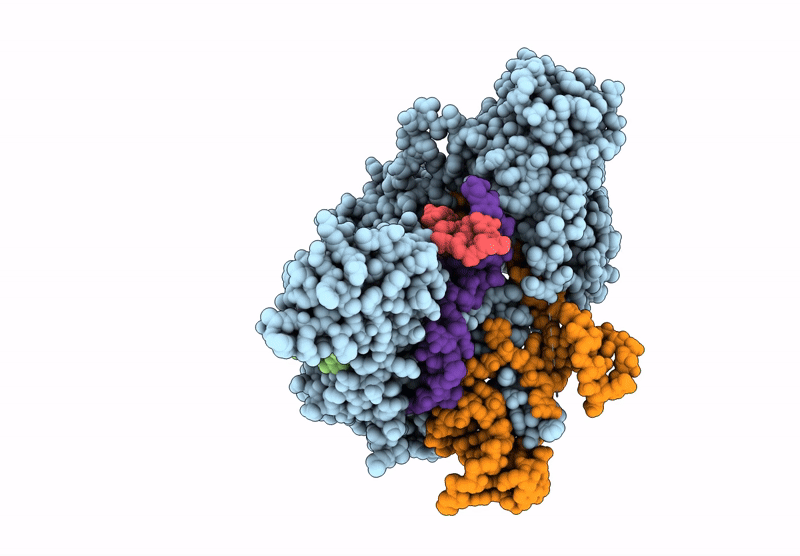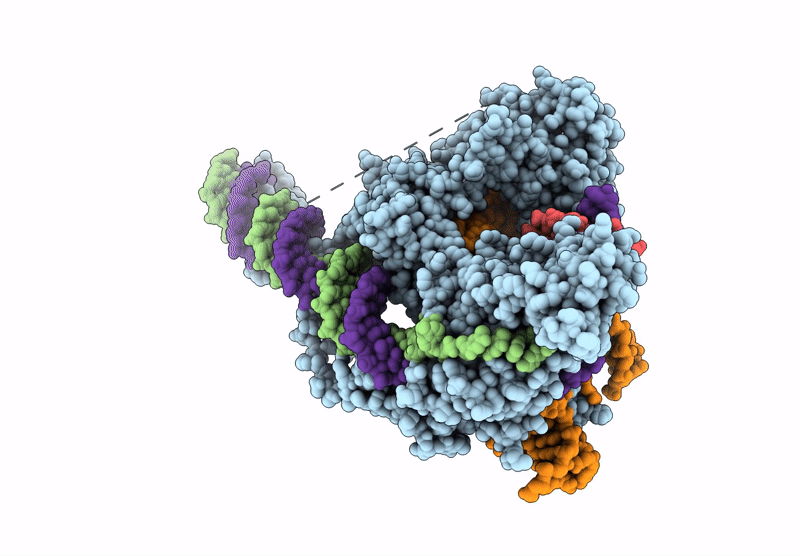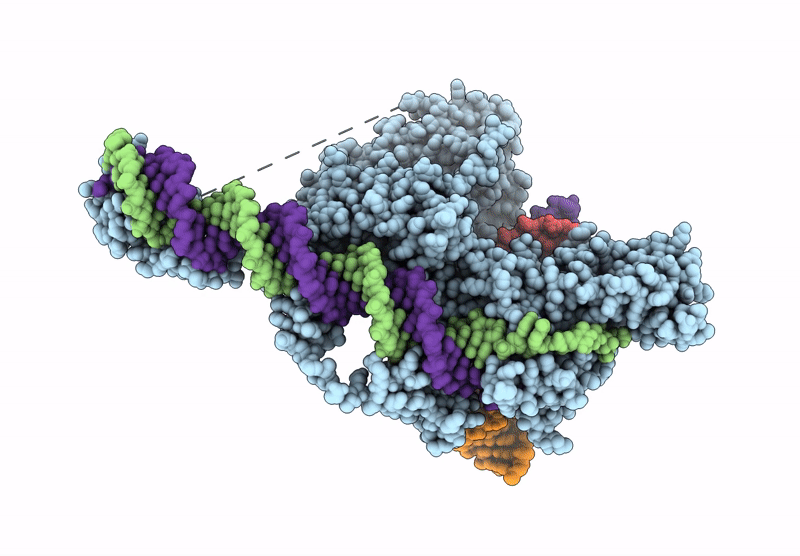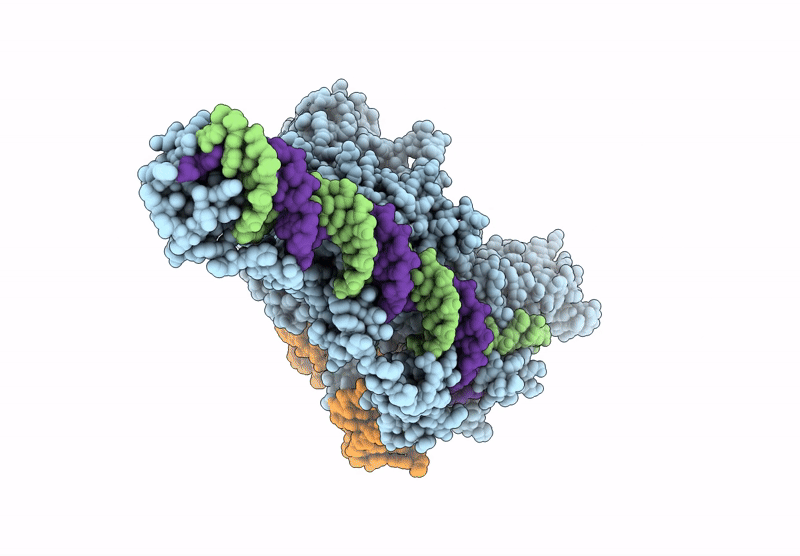
Structure of R2 retrotransposon protein from Taeniopygia guttata initiating target-primed reverse transcription
Biological Source:
Source Organism(s):
Taeniopygia guttata (Taxon ID: 59729)
synthetic construct (Taxon ID: 32630)
synthetic construct (Taxon ID: 32630)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
3.20 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE