Deposition Date
2025-02-05
Release Date
2025-07-30
Last Version Date
2025-07-30
Entry Detail
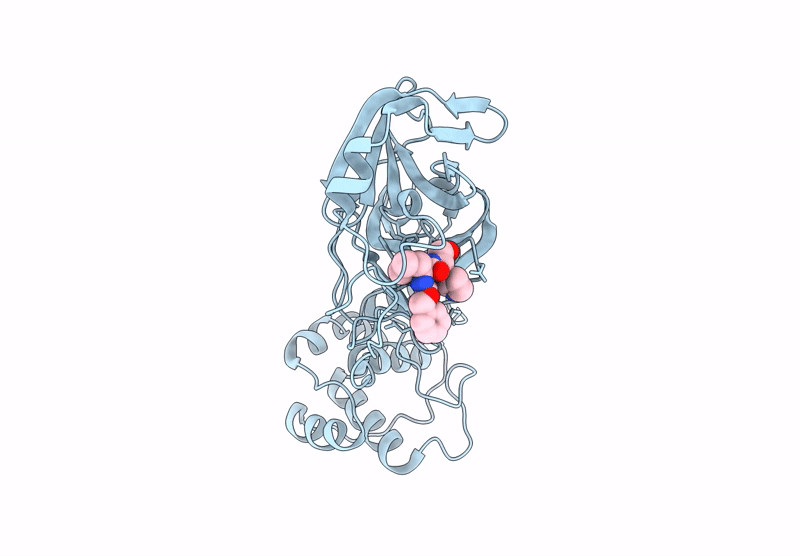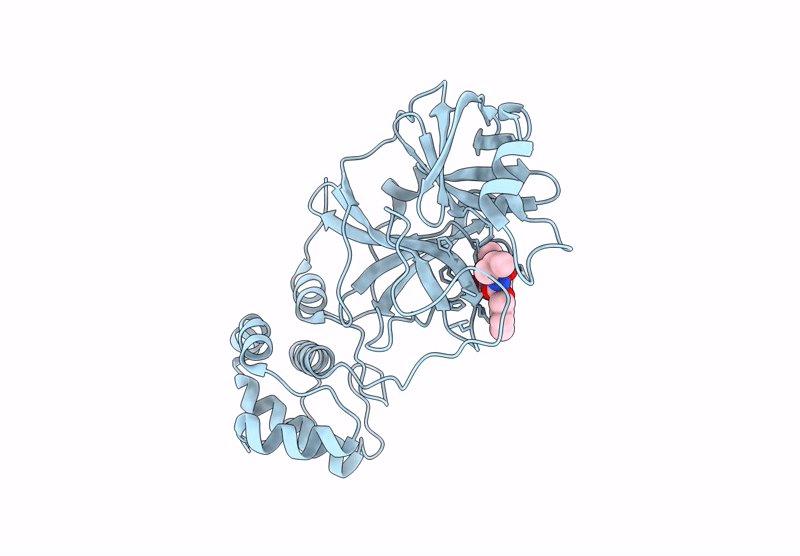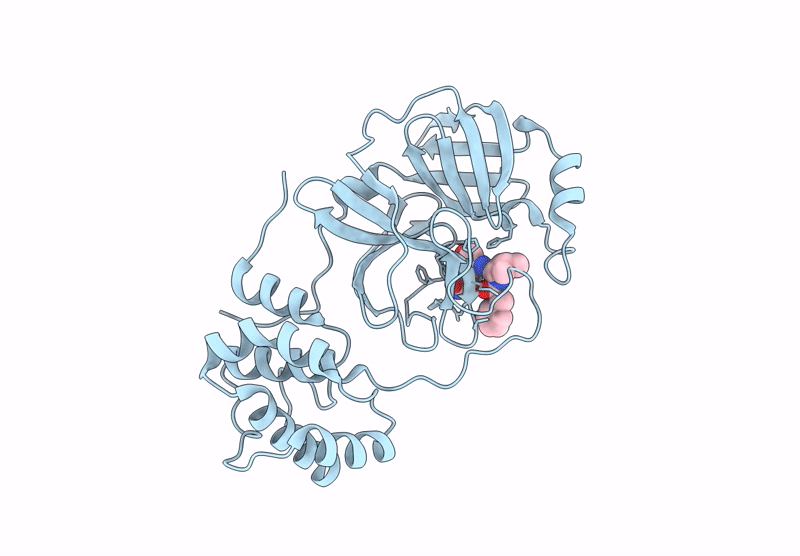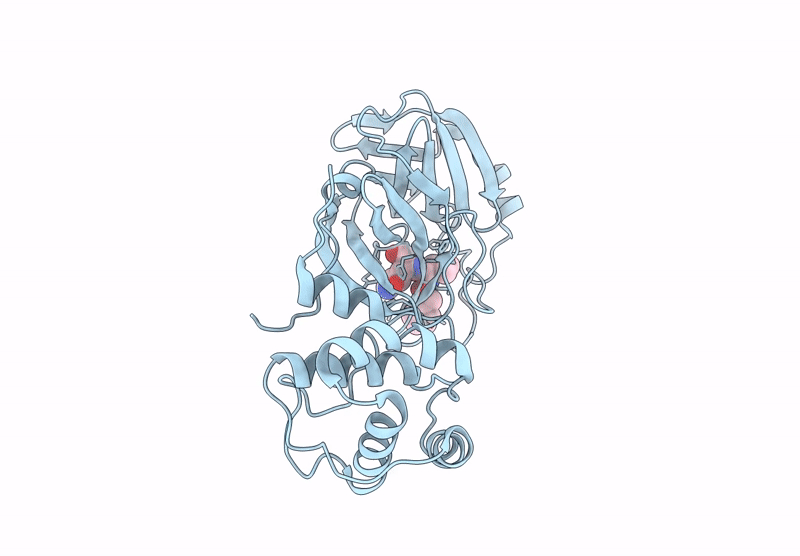
PDB ID:
9N6L
Keywords:
Title:
Room Temperature X-Ray Structure of SARS-CoV-2 Main Protease Mutant D48Y, P168 Deletion in Complex with GC373
Biological Source:
Source Organism(s):
Expression System(s):
Method Details:
Experimental Method:
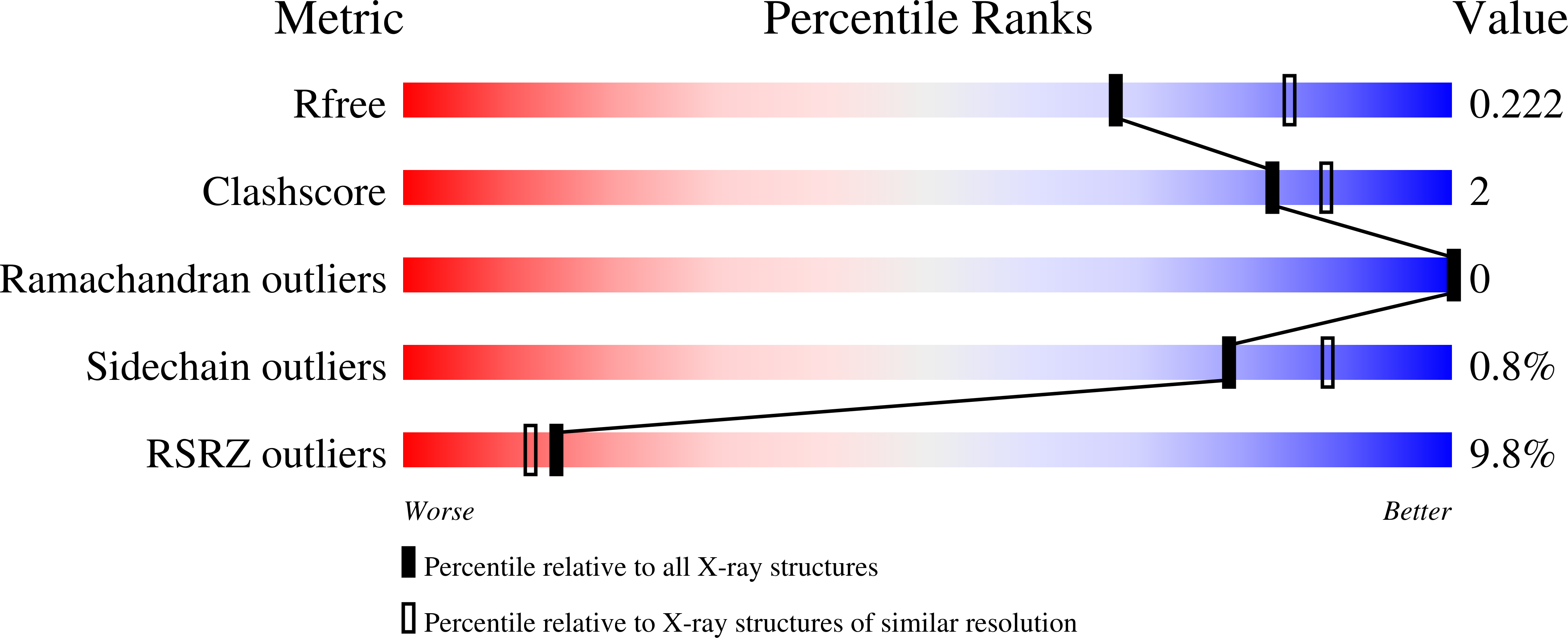
Resolution:
2.20 Å
R-Value Free:
0.22
R-Value Work:
0.18
R-Value Observed:
0.18
Space Group:
I 1 2 1