Deposition Date
2025-02-03
Release Date
2025-09-24
Last Version Date
2025-09-24
Entry Detail
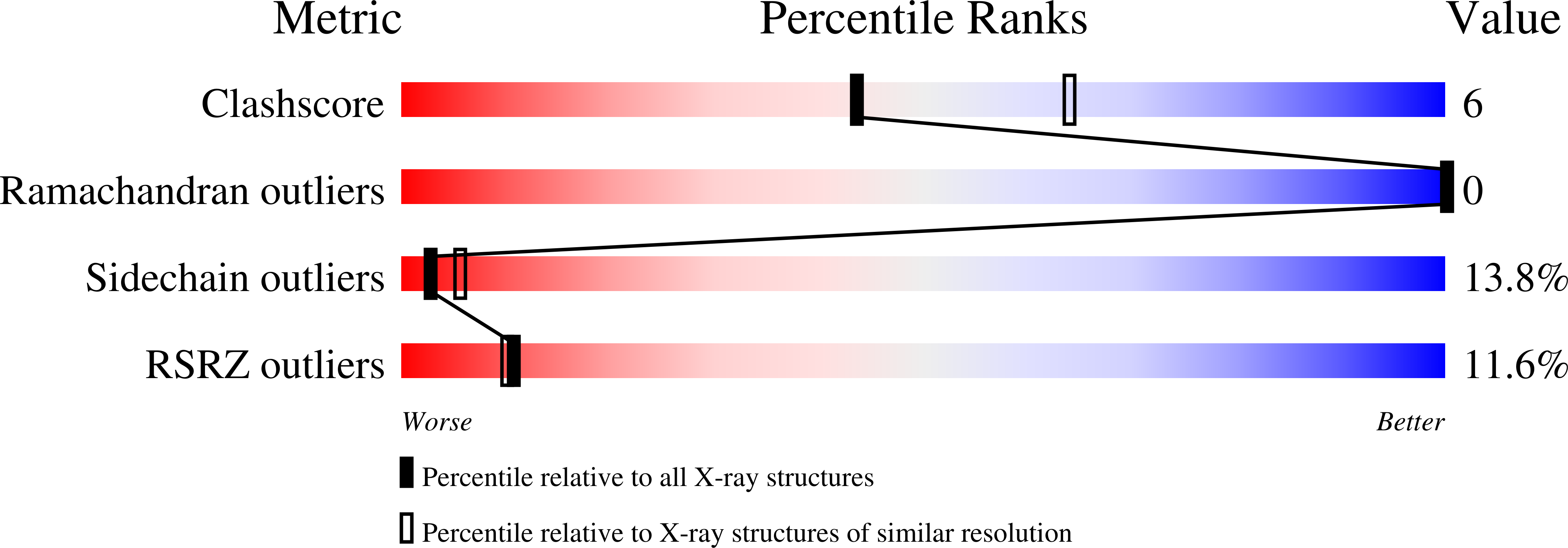
PDB ID:
9N50
Keywords:
Title:
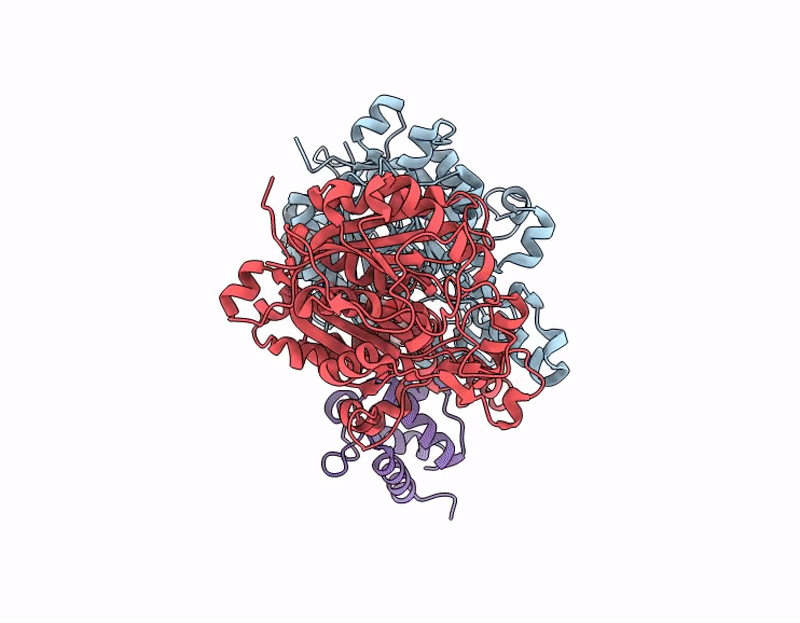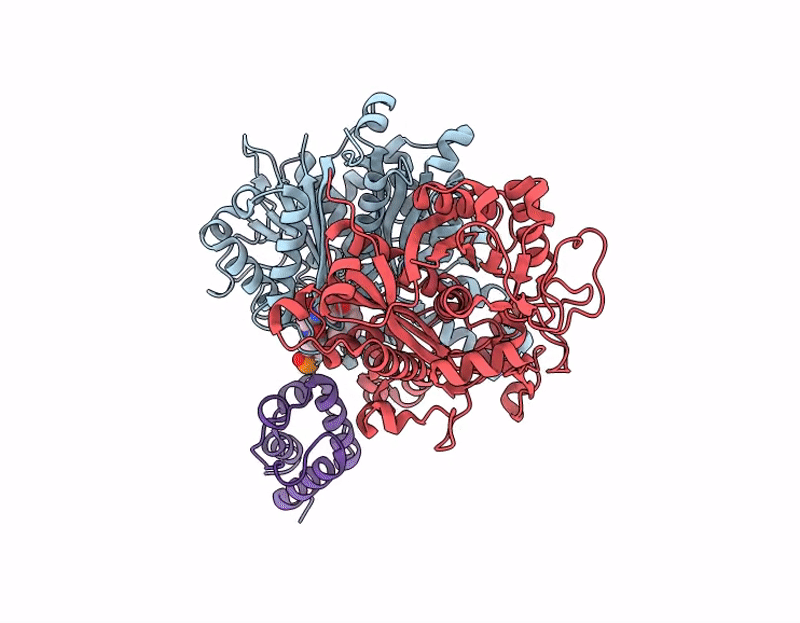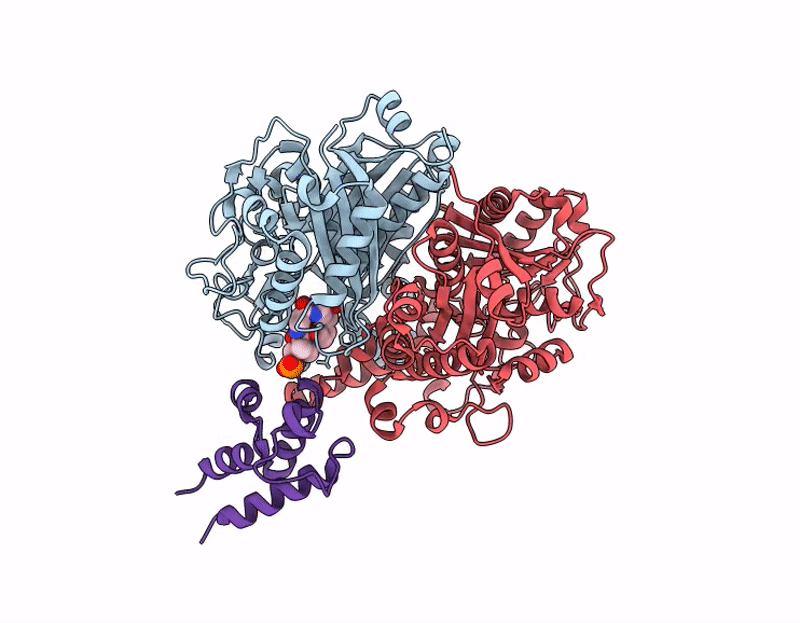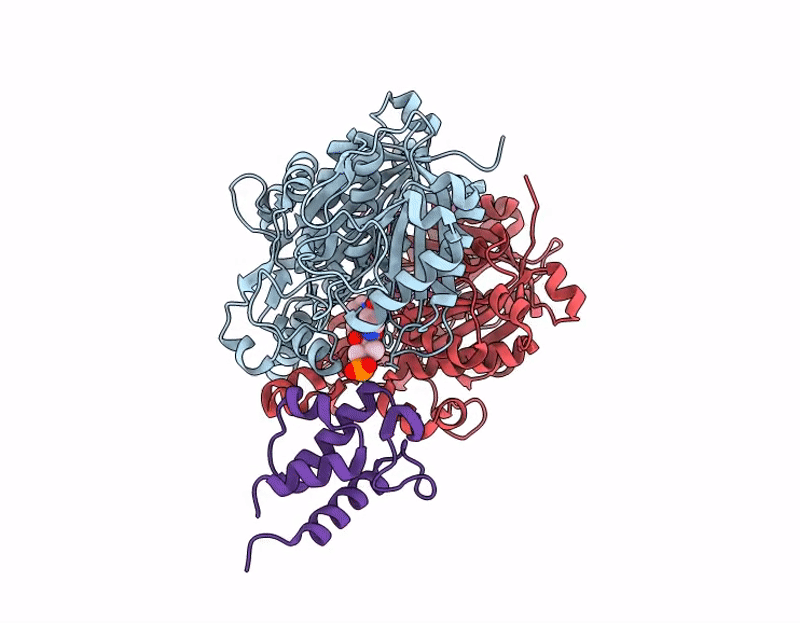
Crosslinked Crystal Structure of Human Mitochondrial Ketosynthase, OXSM, and Crosslinker-crypto Human Mitochondrial Acyl Carrier Protein, C8aBr-mACP
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Expression System(s):
Method Details: