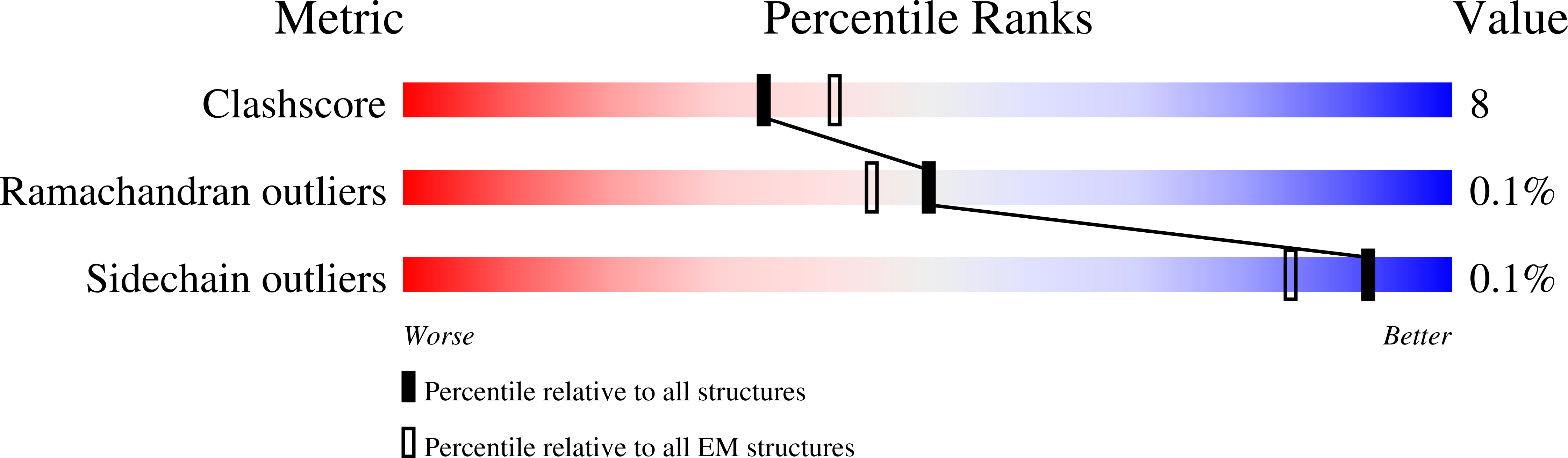
Deposition Date
2025-02-03
Release Date
2025-07-16
Last Version Date
2025-09-17
Entry Detail
PDB ID:
9N4Y
Keywords:
Title:
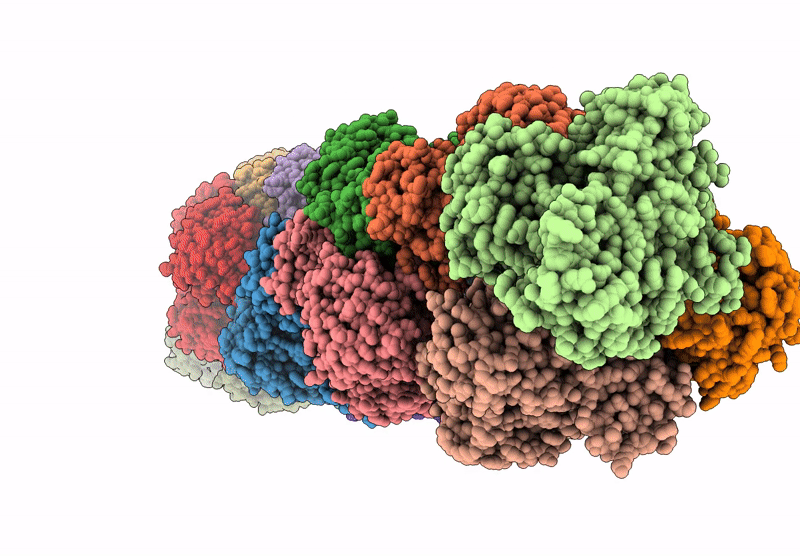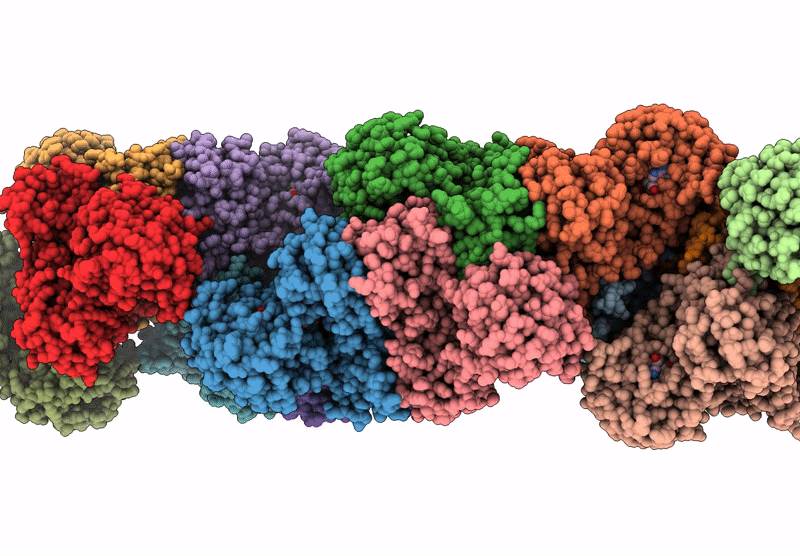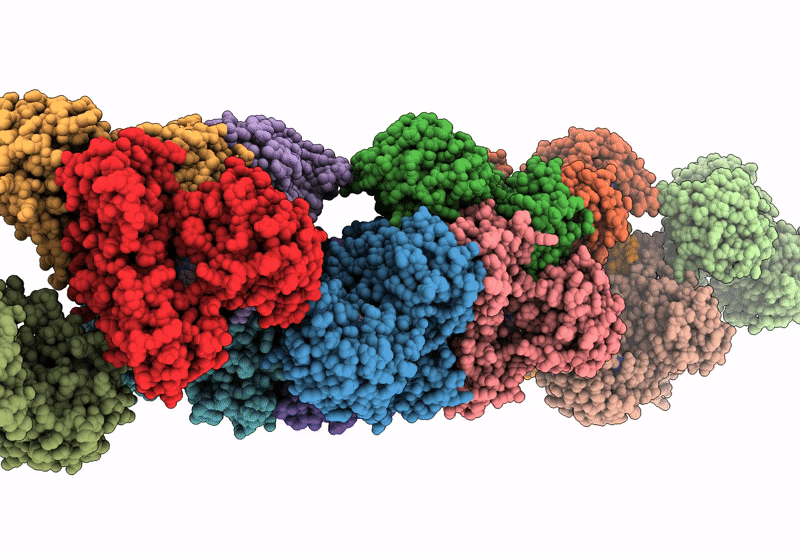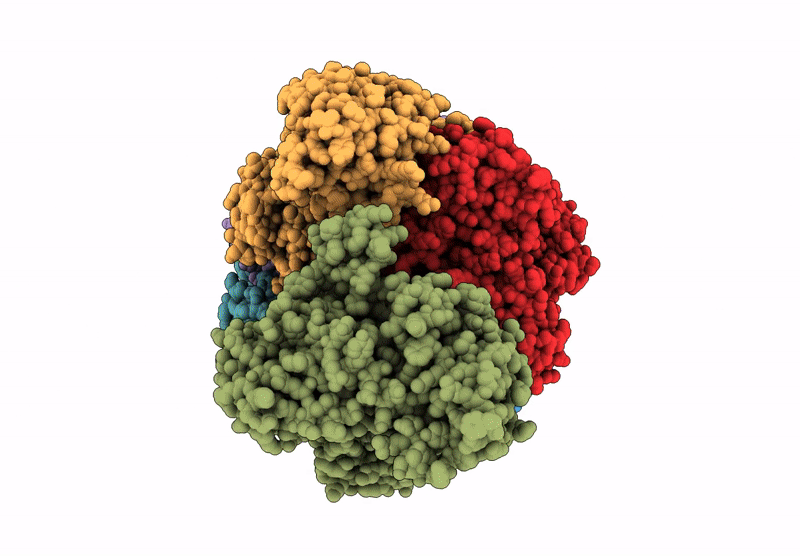
CryoEM structure of a filamentous soluble pyridine nucleotide transhydrogenase
Biological Source:
Source Organism(s):
Azotobacter vinelandii (Taxon ID: 322710)
Method Details:
Experimental Method:
Resolution:
3.70 Å
Aggregation State:
HELICAL ARRAY
Reconstruction Method:
HELICAL