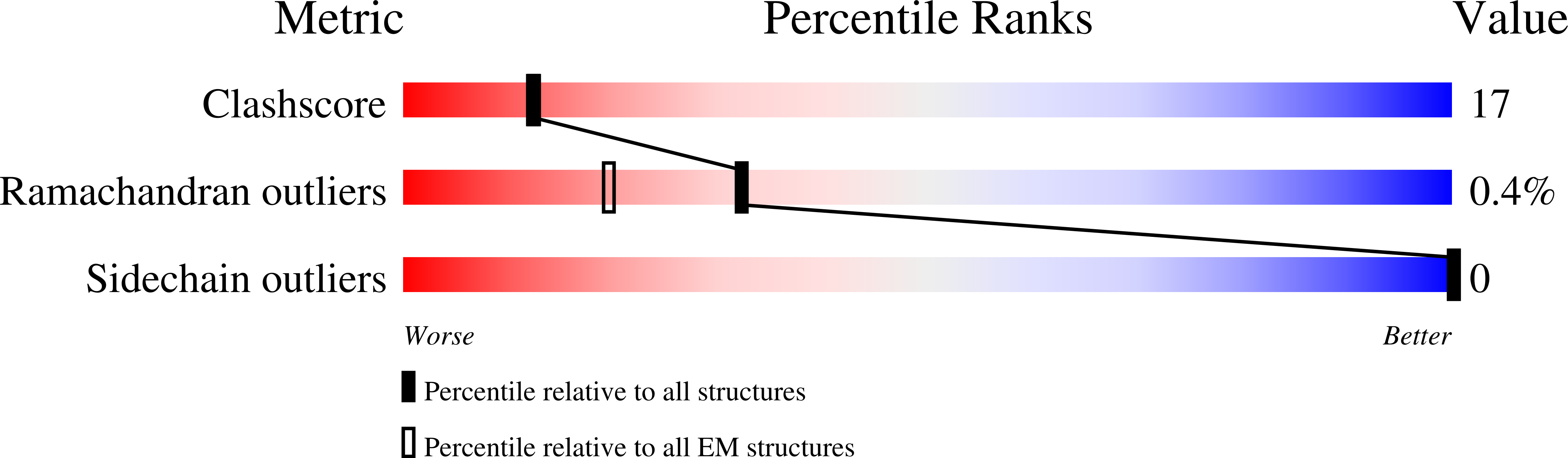
Deposition Date
2025-01-21
Release Date
2025-08-13
Last Version Date
2025-08-13
Entry Detail
PDB ID:
9MY8
Keywords:
Title:
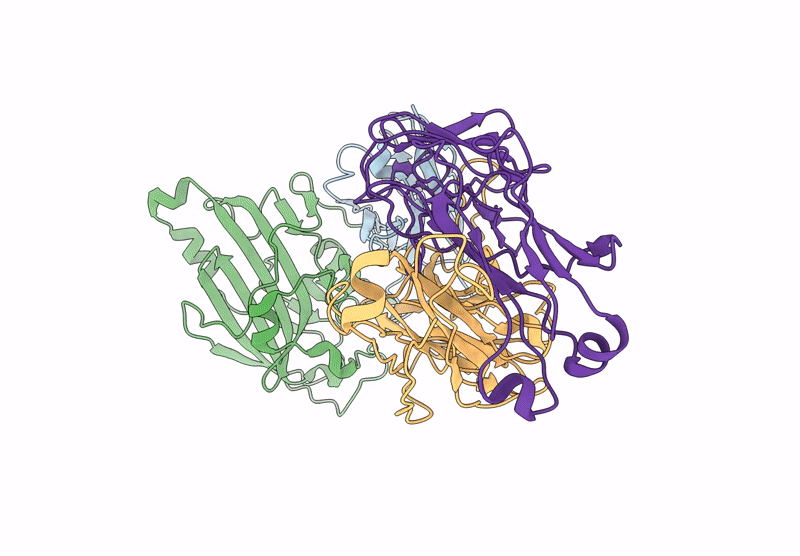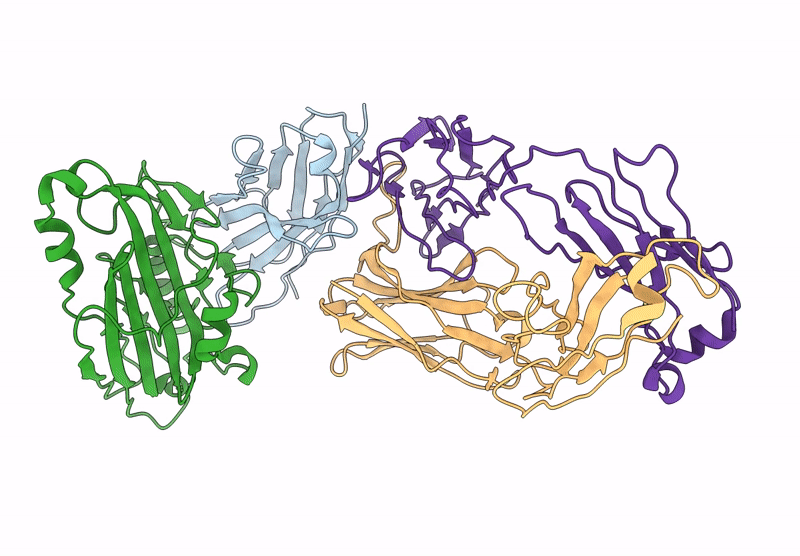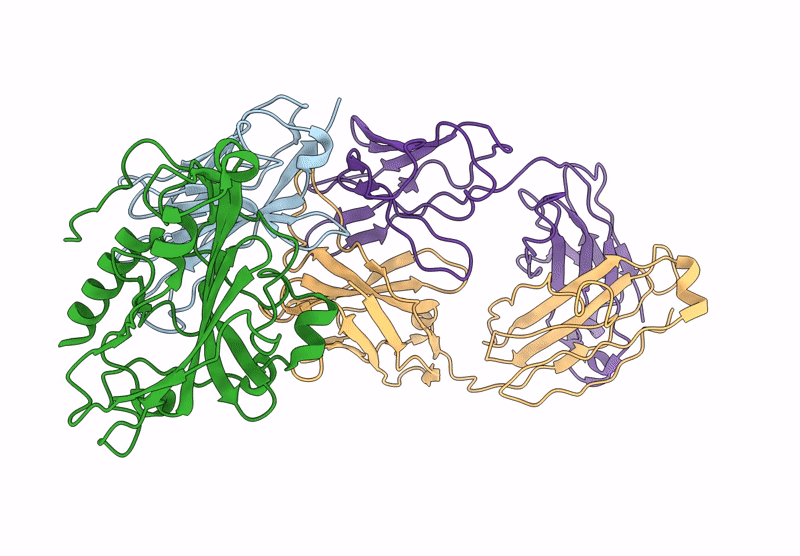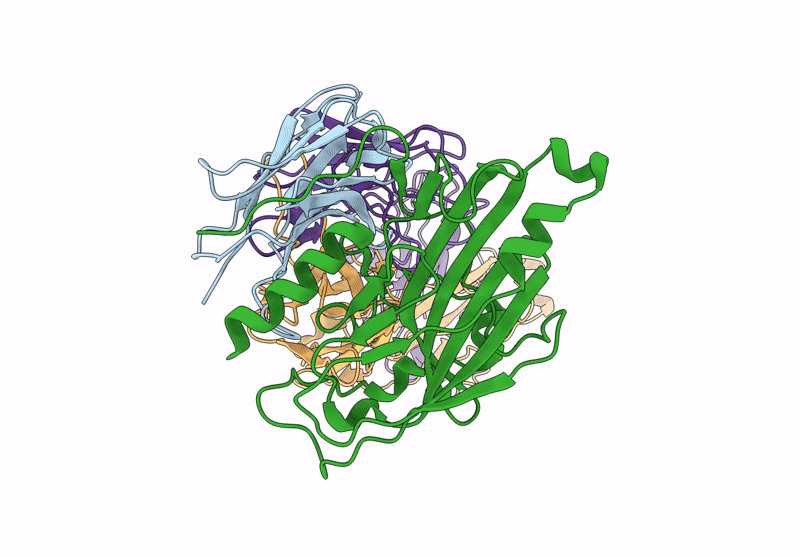
D7 Herpes Virus Simplex Neutralizing Nanobody Bound to HSV Glycoprotein gD
Biological Source:
Source Organism(s):
Lama glama (Taxon ID: 9844)
Homo sapiens (Taxon ID: 9606)
Human herpesvirus 2 (Taxon ID: 10310)
Homo sapiens (Taxon ID: 9606)
Human herpesvirus 2 (Taxon ID: 10310)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
2.30 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE