Deposition Date
2025-03-02
Release Date
2025-12-24
Last Version Date
2026-01-07
Entry Detail
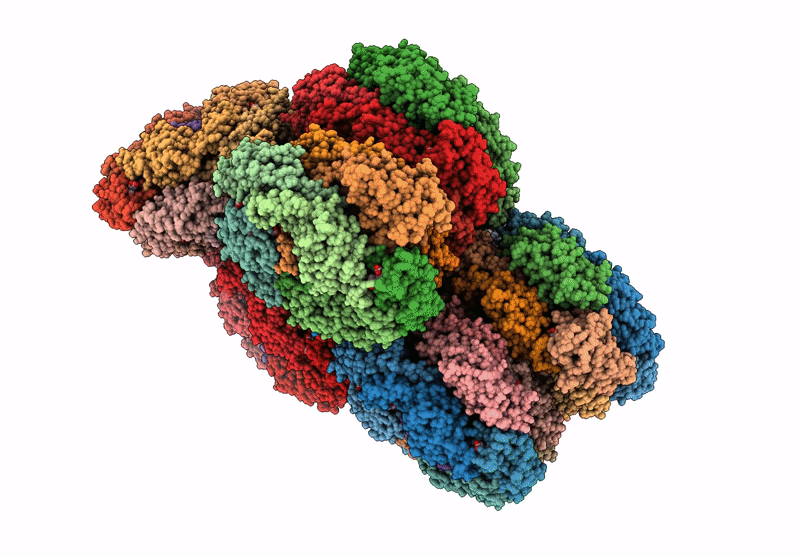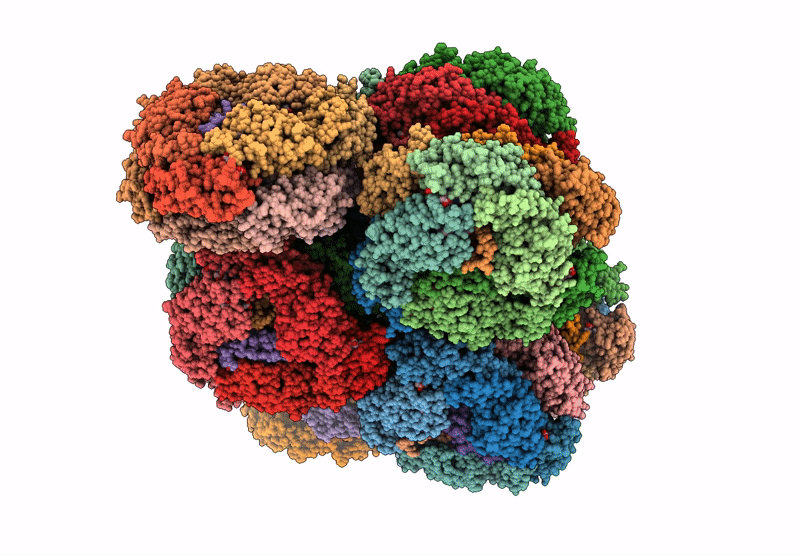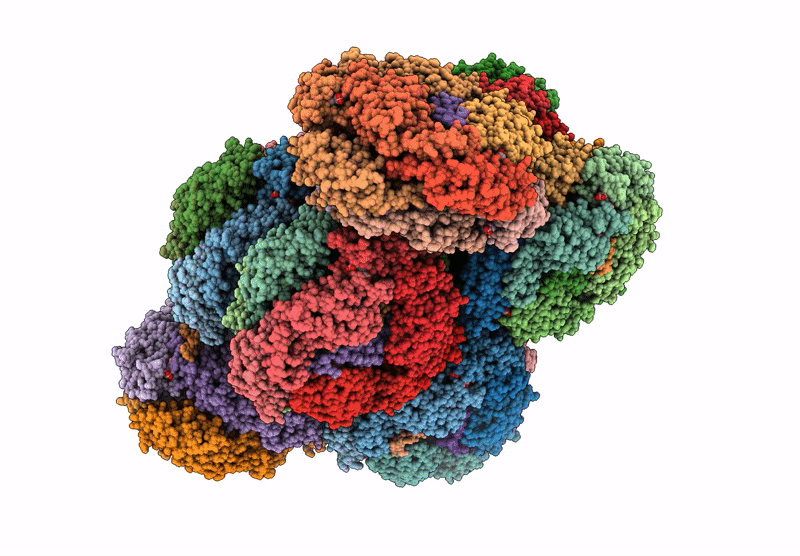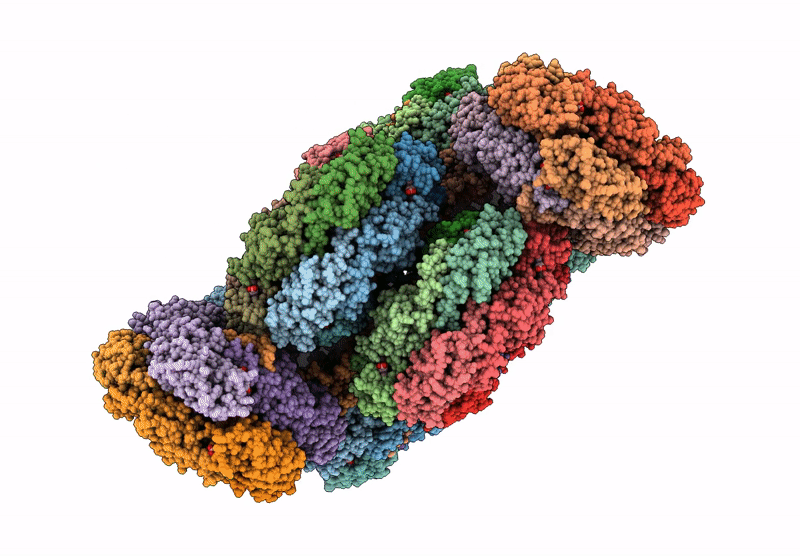
PDB ID:
9M3J
Keywords:
Title:
structure of bundle-shaped PBS with both long rod and (ApcA2B3ApcD) trimer
Biological Source:
Source Organism(s):
Gloeobacter violaceus PCC 7421 (Taxon ID: 251221)
Method Details:
Experimental Method:
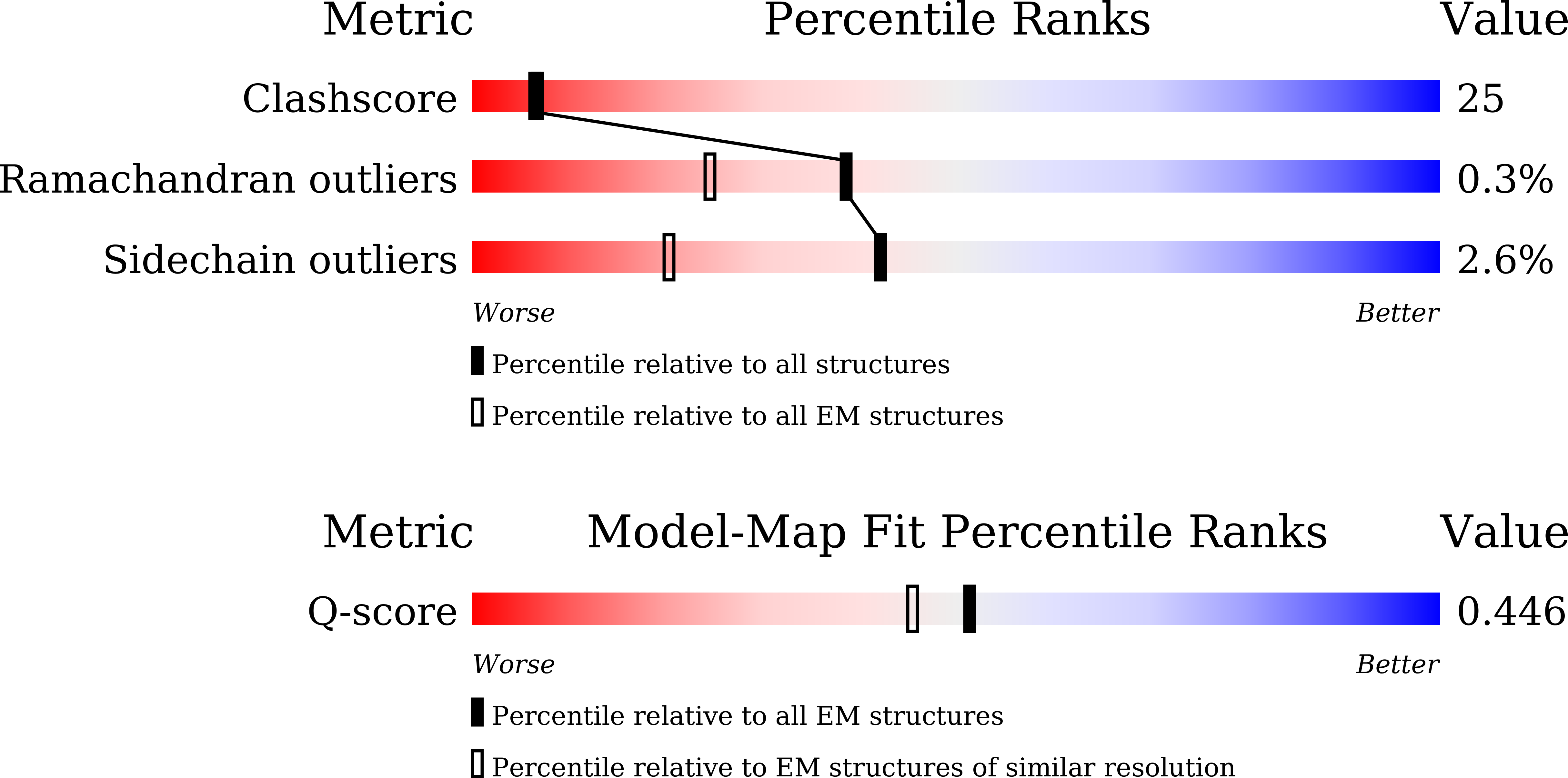
Resolution:
3.37 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE