Deposition Date
2025-02-13
Release Date
2025-04-16
Last Version Date
2025-04-30
Entry Detail
PDB ID:
9LW6
Keywords:
Title:
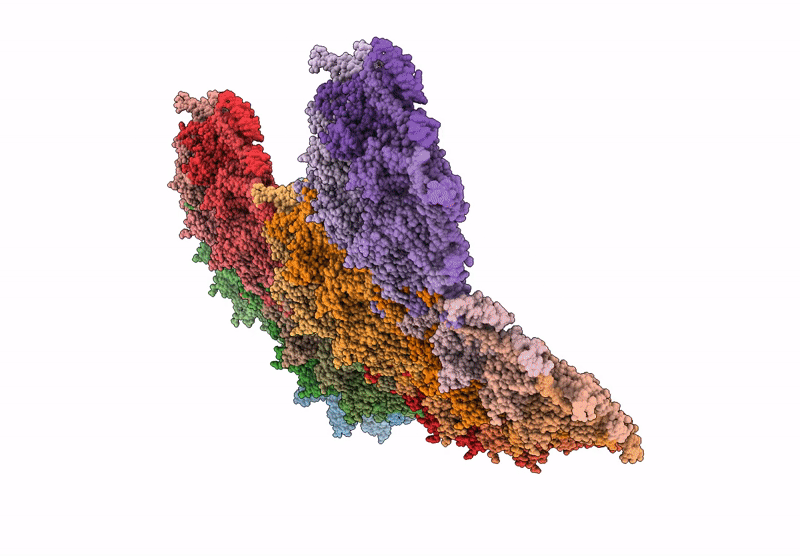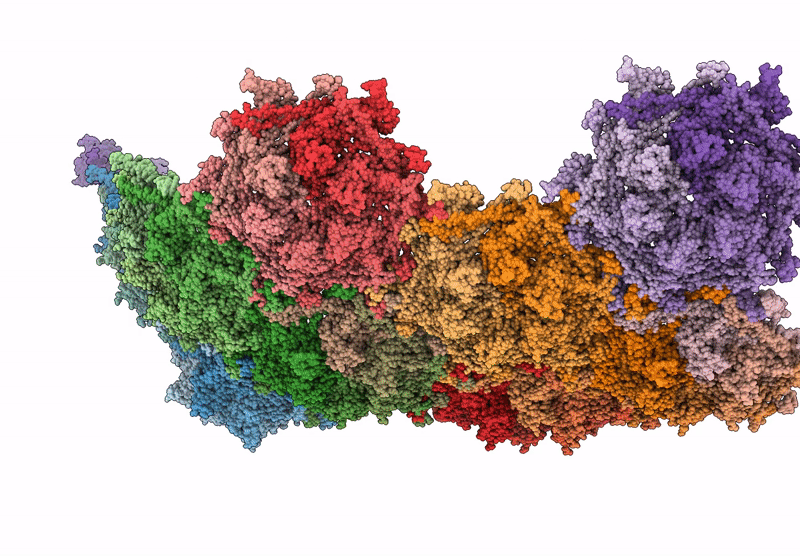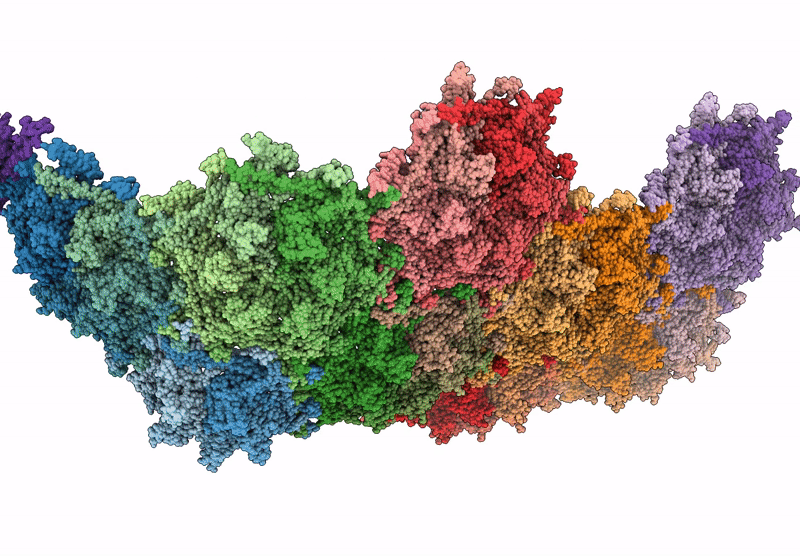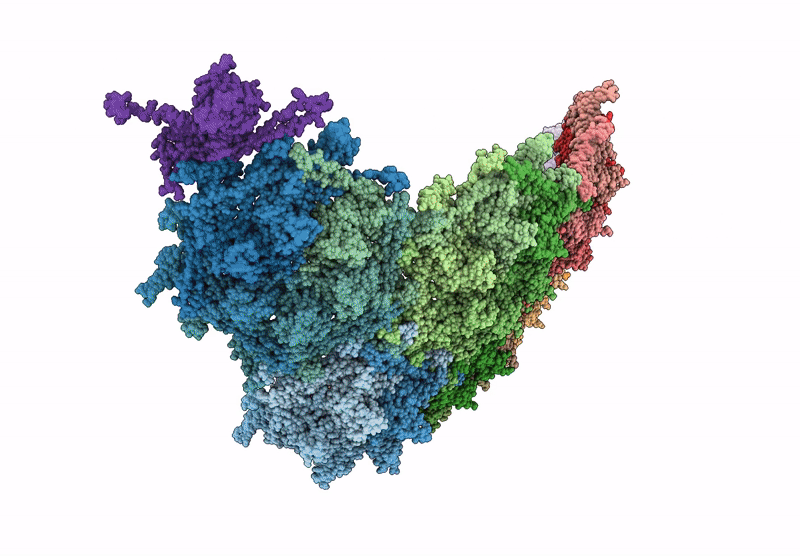
Top cap of bacteriophage Mycofy1 mature head (C5 symmetry)
Biological Source:
Source Organism(s):
Mycolicibacterium phage Mycofy1 (Taxon ID: 3349809)
Method Details:
Experimental Method:
Resolution:
3.42 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE


