Deposition Date
2025-01-16
Release Date
2025-12-17
Last Version Date
2026-01-14
Entry Detail
PDB ID:
9LKL
Keywords:
Title:
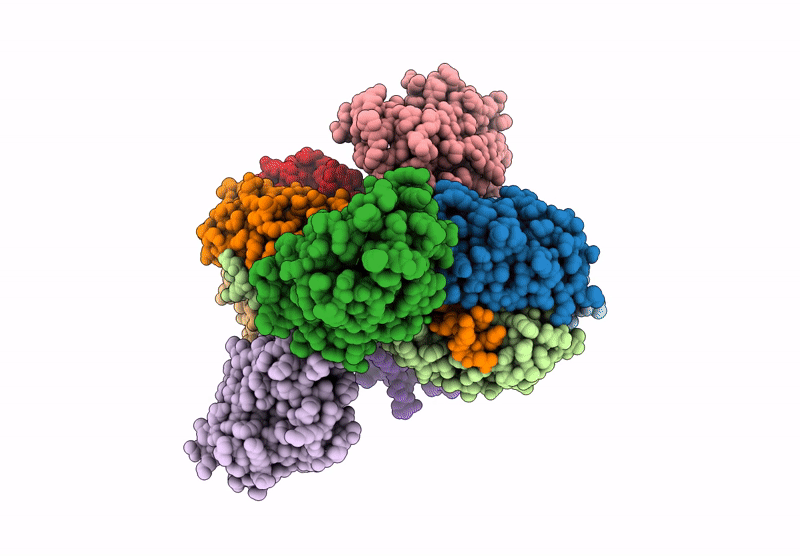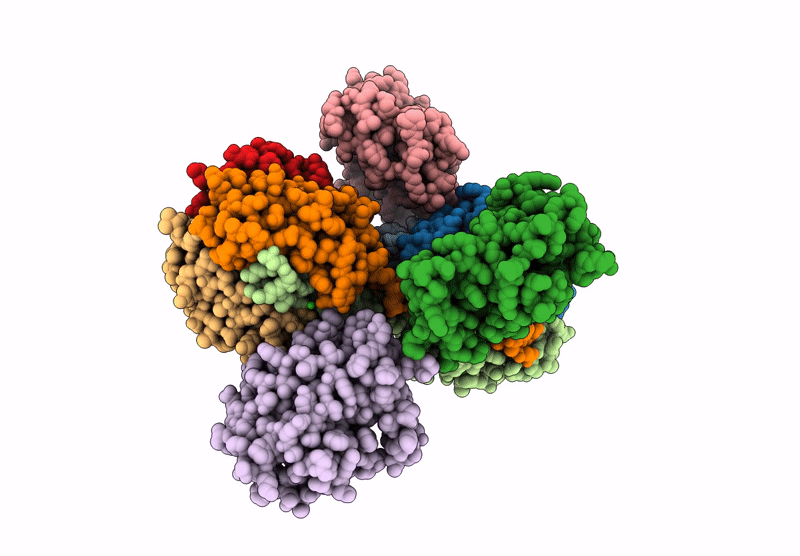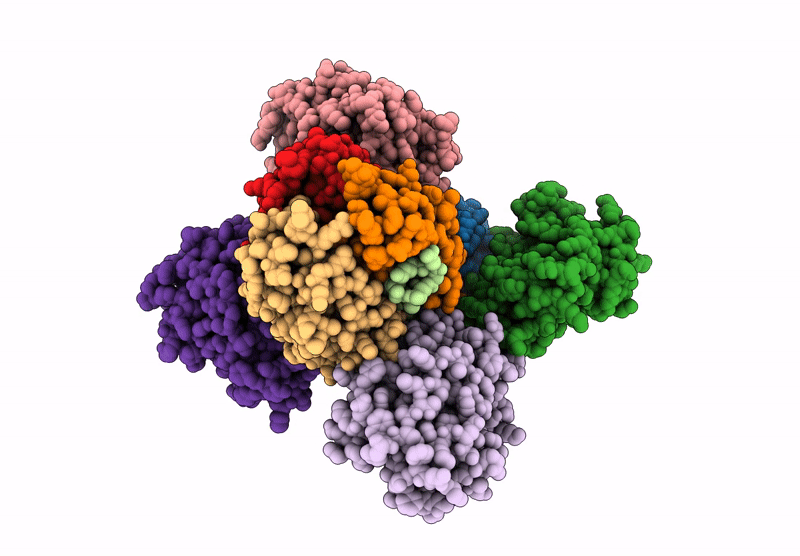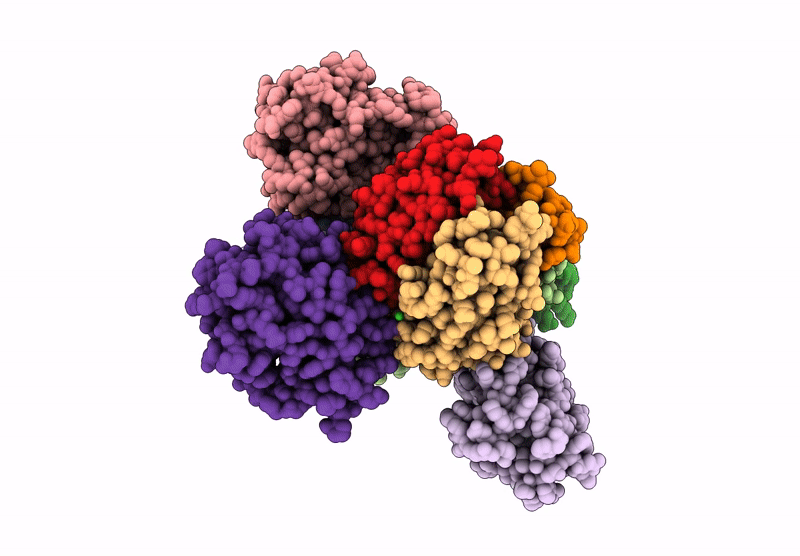
Cryo-EM map of C1ql1-gC1q hexamer and BAI3-eCUB complex
Biological Source:
Source Organism(s):
Mus musculus (Taxon ID: 10090)
Homo sapiens (Taxon ID: 9606)
Homo sapiens (Taxon ID: 9606)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
3.48 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE


