Abstact
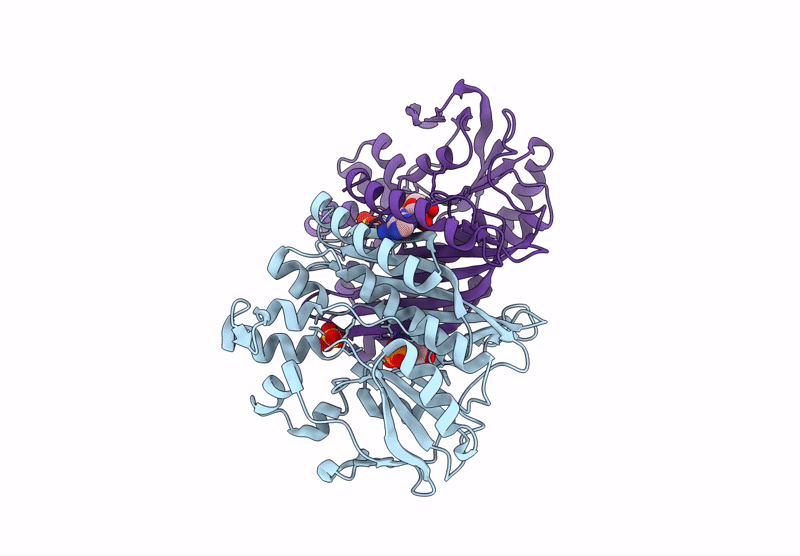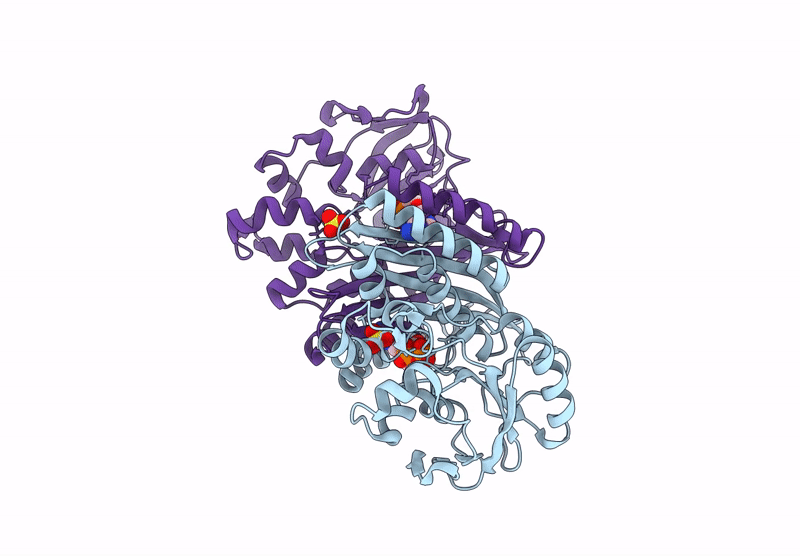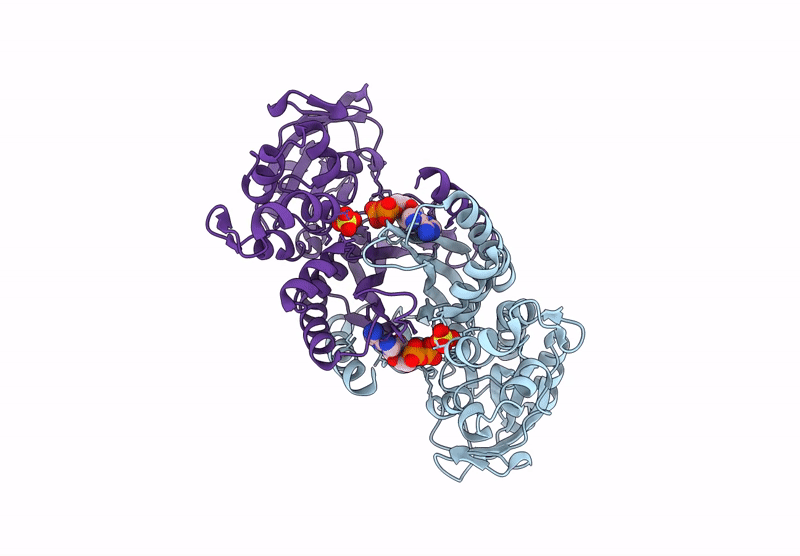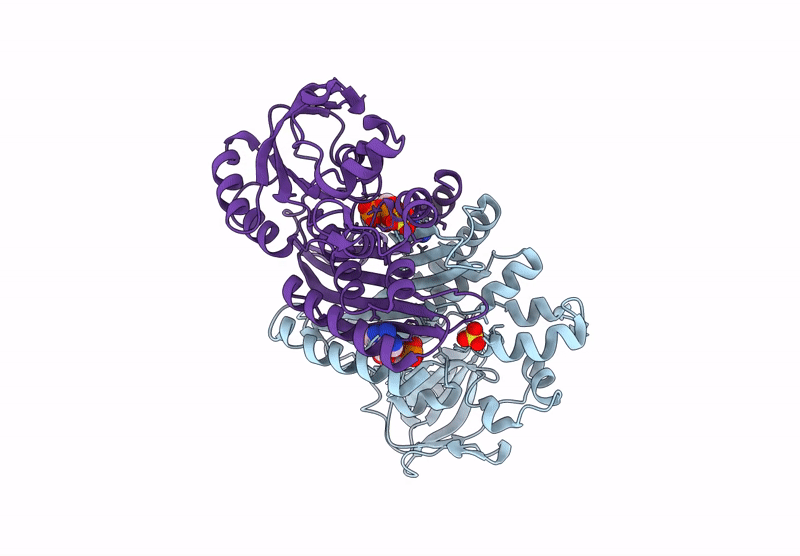
The enzyme 5-aminoimidazole ribonucleotide synthetase (AIR synthetase, AIRS, known also as PurM) catalyzes the fifth step in the de novo purine biosynthetic pathway: the ATP-dependent conversion of formylglycinamidine ribonucleotide (FGAM) to aminoimidazole ribonucleotide (AIR). AIRS belongs to the PurM superfamily, sharing ATPase domains. In this study, we employed hyperthermostable AIRS from Pyrococcus abyssi (Pa) and Pyrococcus horikoshii (Ph), members of the thermophilic archaeal genus known for extreme heat tolerance, to resolve the substrate-binding site and structural features. Crystal structures of AIRS were determined in complex with the substrate analog formylglycinamide ribonucleotide (FGAR), the non-hydrolyzable ATP analog adenylyl-imidodiphosphate (AMPPNP), the product ADP, and metal cofactors (Mn2+, Mg2+, and K+), respectively. AMPPNP and FGAR bind to the α1-β1 loop and the disordered N-terminal tail, respectively, stabilizing the active-site conformation. These findings provide a structural framework for understanding substrate positioning and catalysis within AIRS. A unique non-domain-swapping feature observed in the first 54 residues of the N-terminal domain in PaAIRS and PhAIRS, differing from their orthologs. The domain-swappable feature is found to be β1-β2 loop-dependent and can be categorized into two distinct types in the AIRS family: a short-looped, non-swapping archaeal type and a long-looped, swapping bacterial type. Structural, mutational, and biophysical analyses suggest that the thermostability is supported by enhanced hydrophobic core packing, strengthened dimer interfaces, and shortened surface loops. Collectively, these findings not only elucidate the substrate binding, the catalytic mechanism, and domain swapping of AIRS but also contribute to our understanding of the compact and robust architecture characteristics of thermophilic proteins.