Deposition Date
2024-09-26
Release Date
2025-10-22
Last Version Date
2026-01-28
Entry Detail
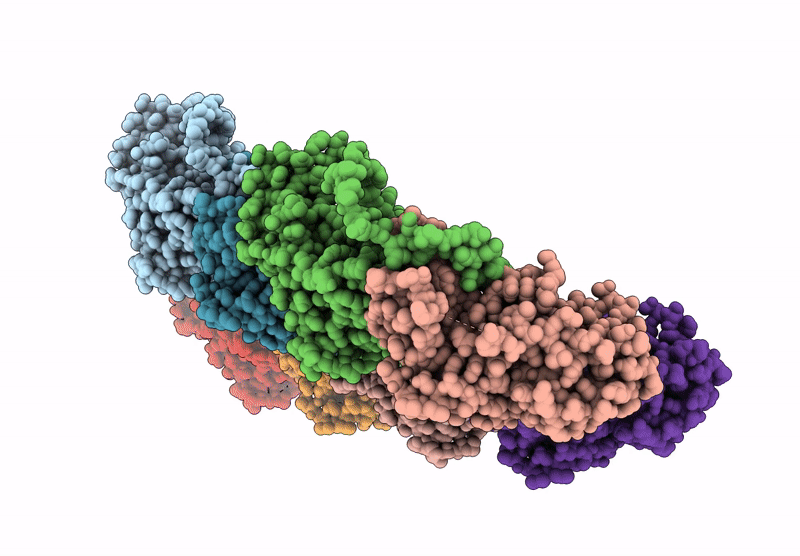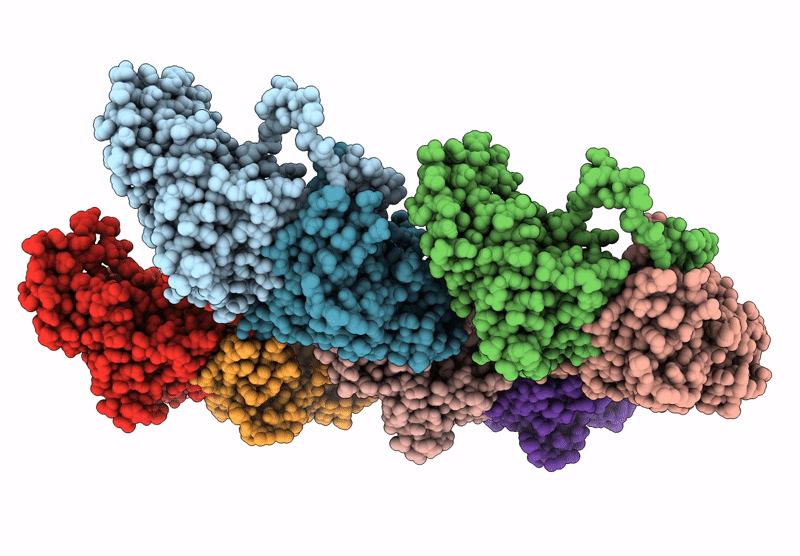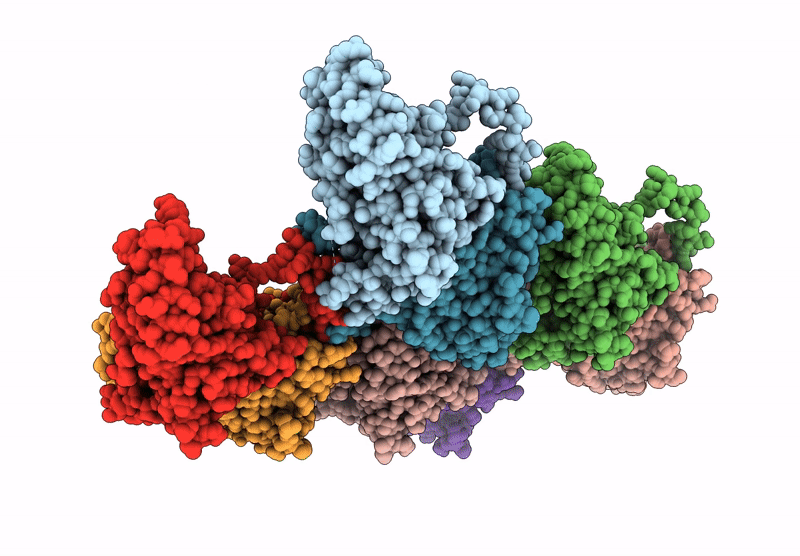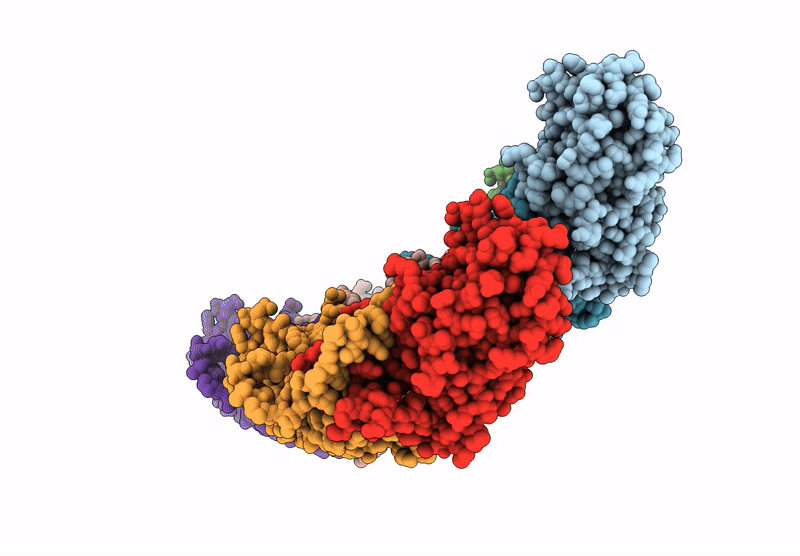
PDB ID:
9JPR
Keywords:
Title:
Local refinement of H11 nanotubes assembled from baculovirus capsid protein
Biological Source:
Source Organism(s):
Schistosoma japonicum (Taxon ID: 6182)
Helicoverpa armigera nucleopolyhedrovirus (Taxon ID: 51313)
Helicoverpa armigera nucleopolyhedrovirus (Taxon ID: 51313)
Expression System(s):
Method Details:
Experimental Method:
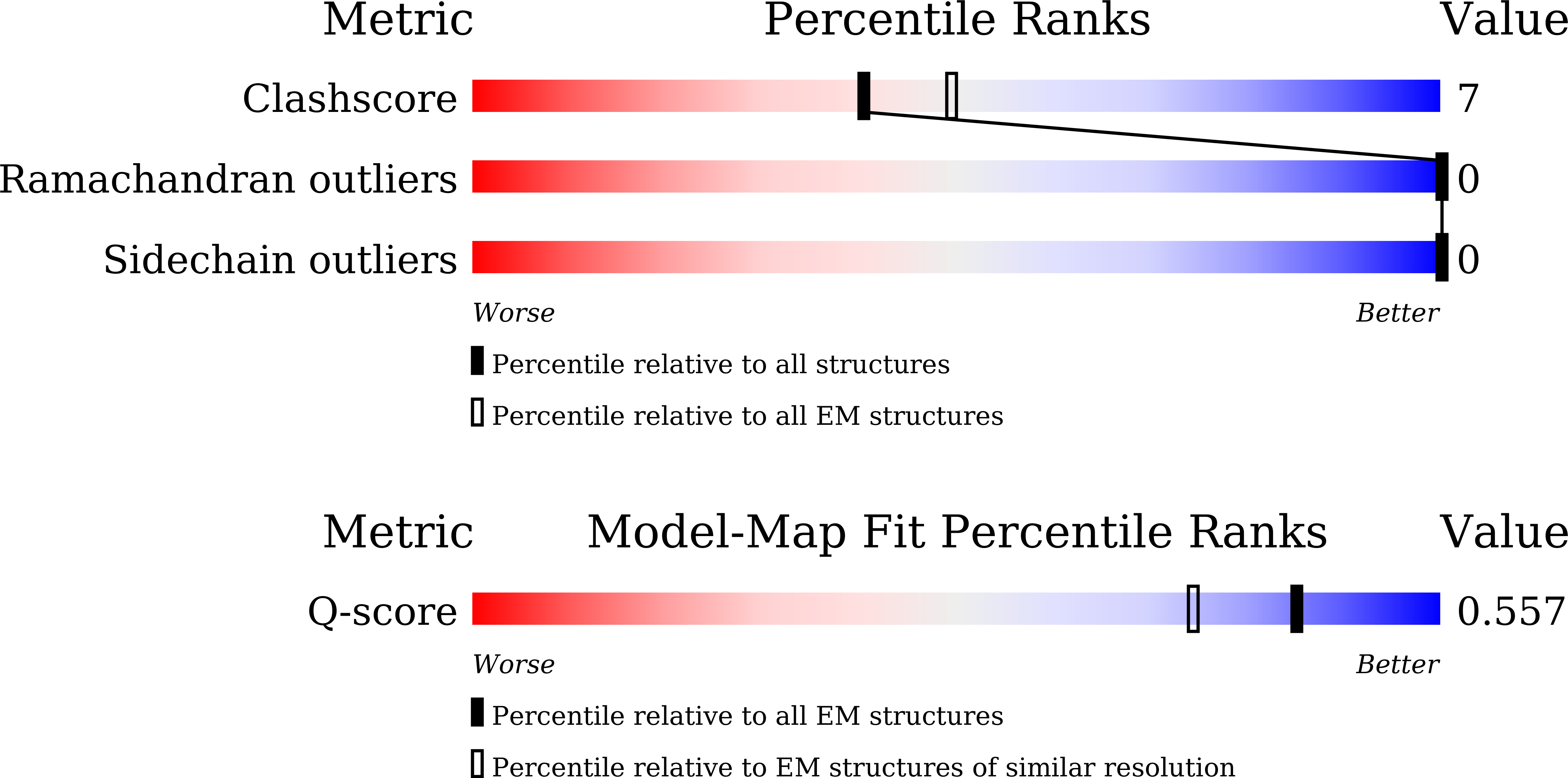
Resolution:
2.80 Å
Aggregation State:
HELICAL ARRAY
Reconstruction Method:
SINGLE PARTICLE