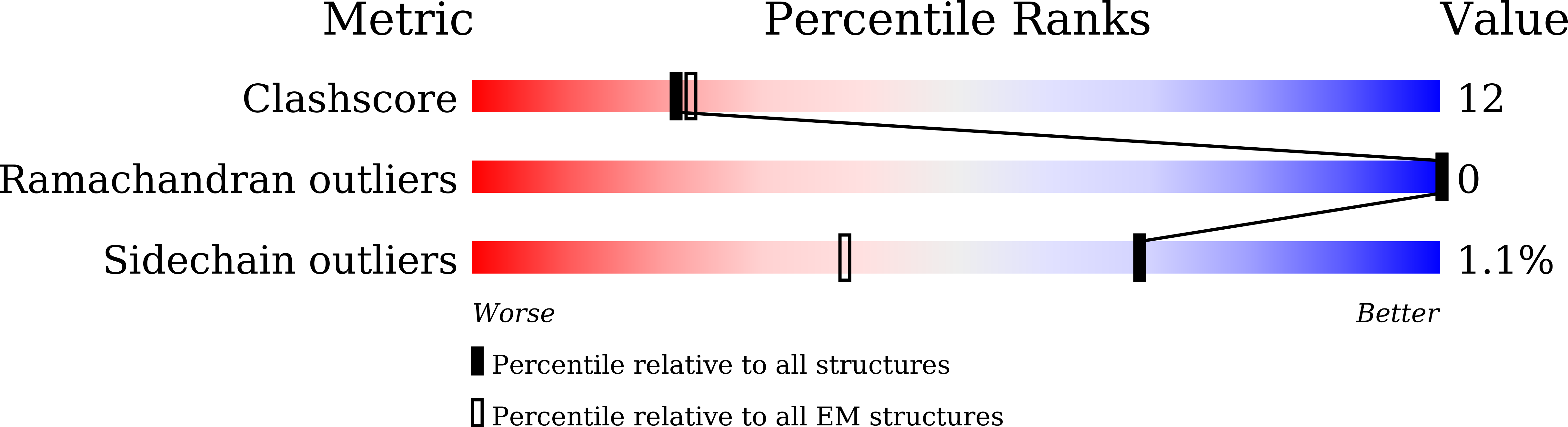
Deposition Date
2024-09-10
Release Date
2025-02-05
Last Version Date
2025-02-05
Entry Detail
PDB ID:
9JHX
Keywords:
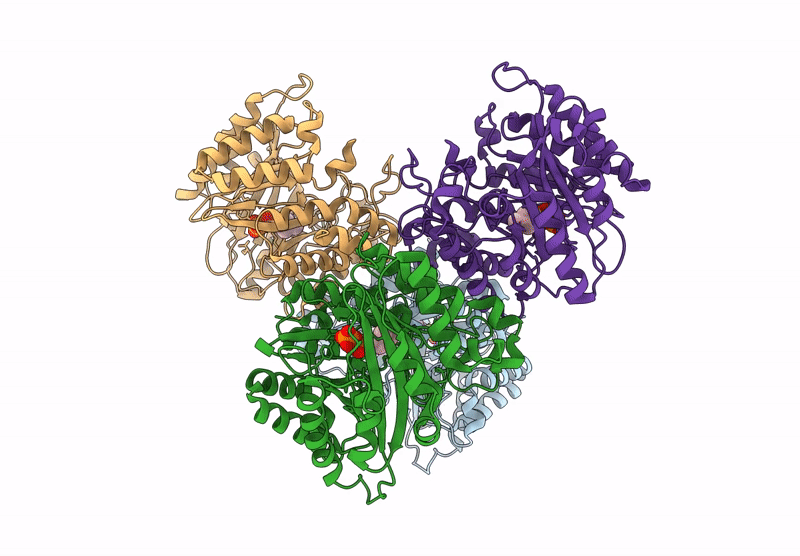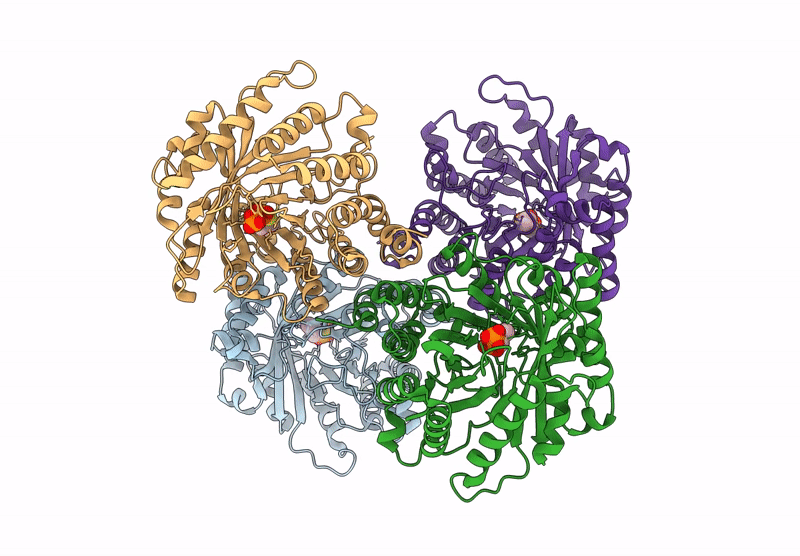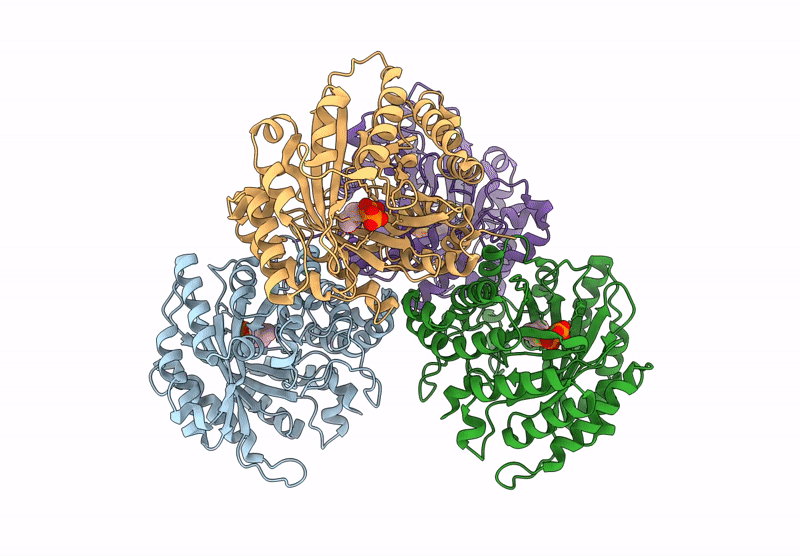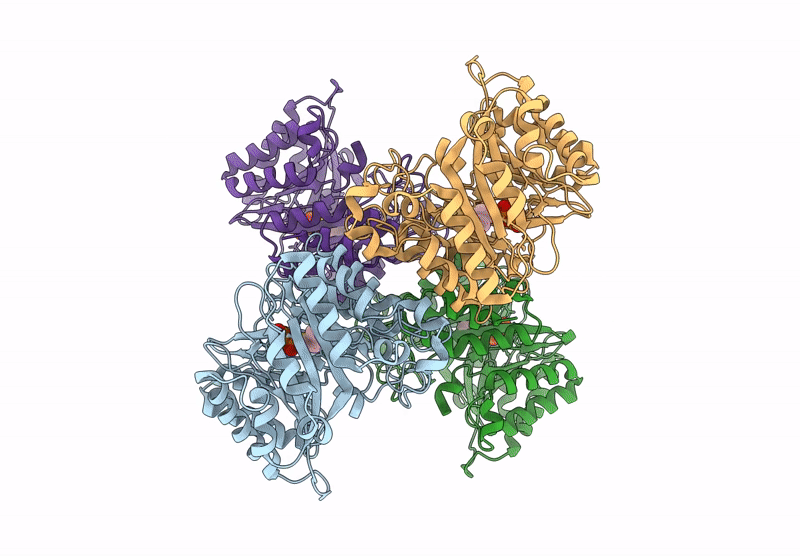
Title:
Versatile Aromatic Prenyltransferase auraA mutant-Y207A in complex with DMSPP
Biological Source:
Source Organism:
Penicillium solitum (Taxon ID: 60172)
Host Organism:
Method Details:
Experimental Method:
Resolution:
2.86 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE