Deposition Date
2024-09-05
Release Date
2025-09-10
Last Version Date
2026-01-21
Entry Detail
PDB ID:
9JFS
Keywords:
Title:
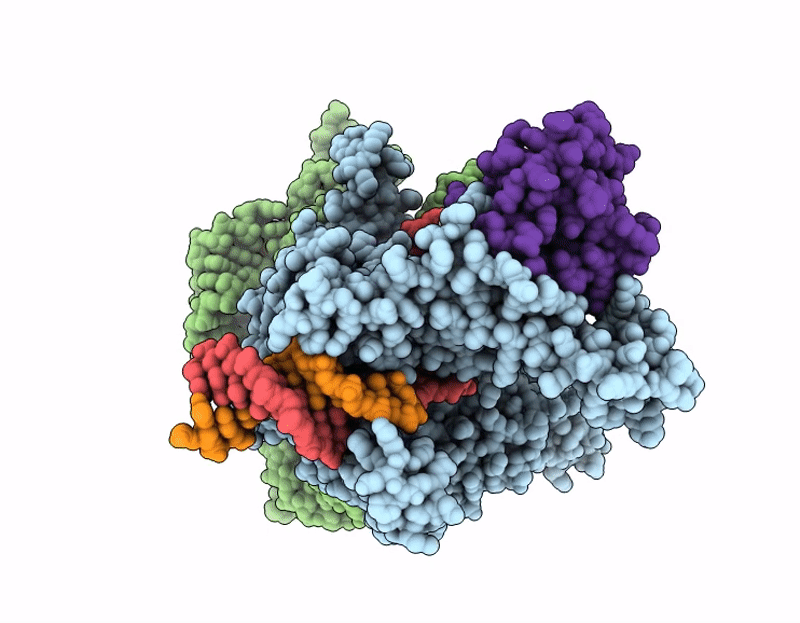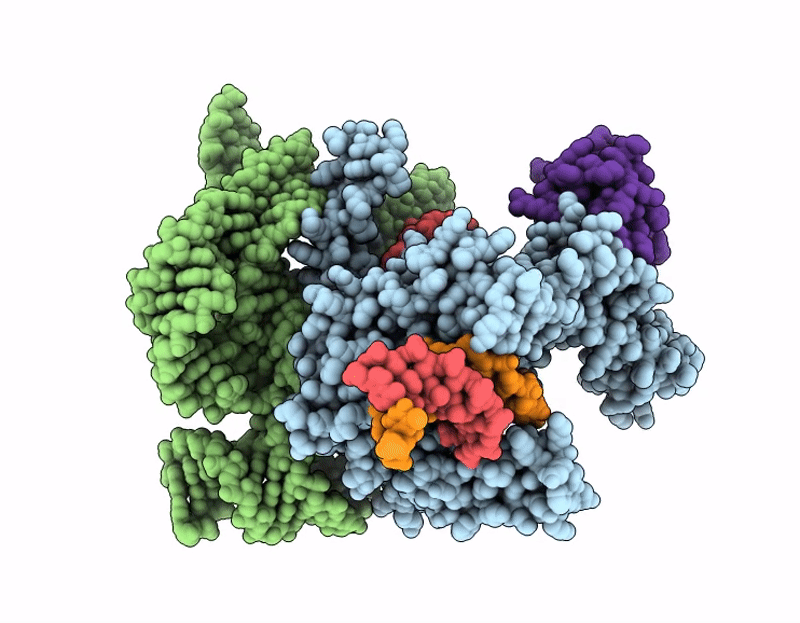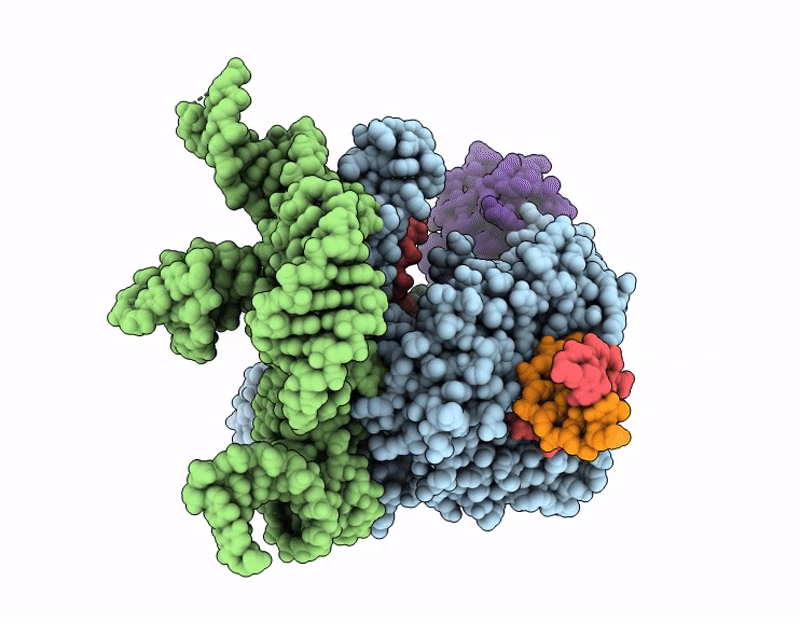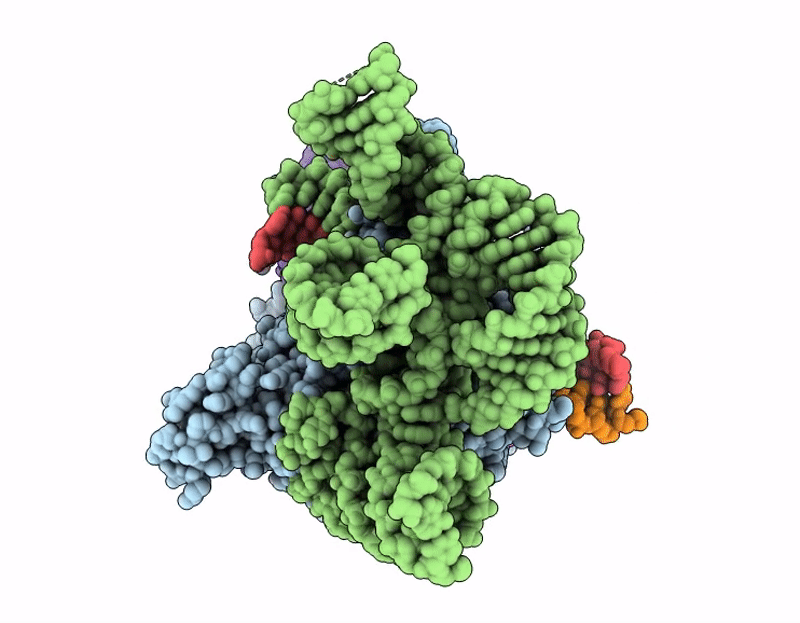
Structure of Cas12p-TrxA-guide RNA-target DNA complex(29nt TS and 11nt NTS)
Biological Source:
Source Organism(s):
unidentified (Taxon ID: 32644)
synthetic construct (Taxon ID: 32630)
Escherichia coli BL21(DE3) (Taxon ID: 469008)
synthetic construct (Taxon ID: 32630)
Escherichia coli BL21(DE3) (Taxon ID: 469008)
Expression System(s):
Method Details:
Experimental Method:
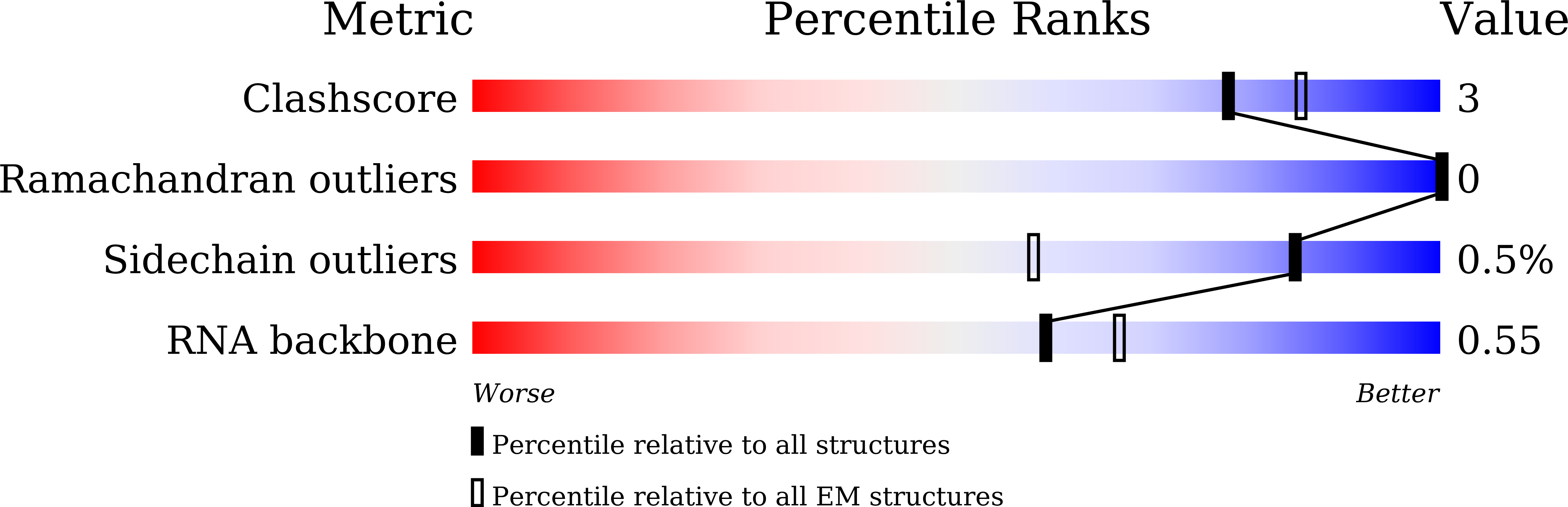
Resolution:
2.67 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE