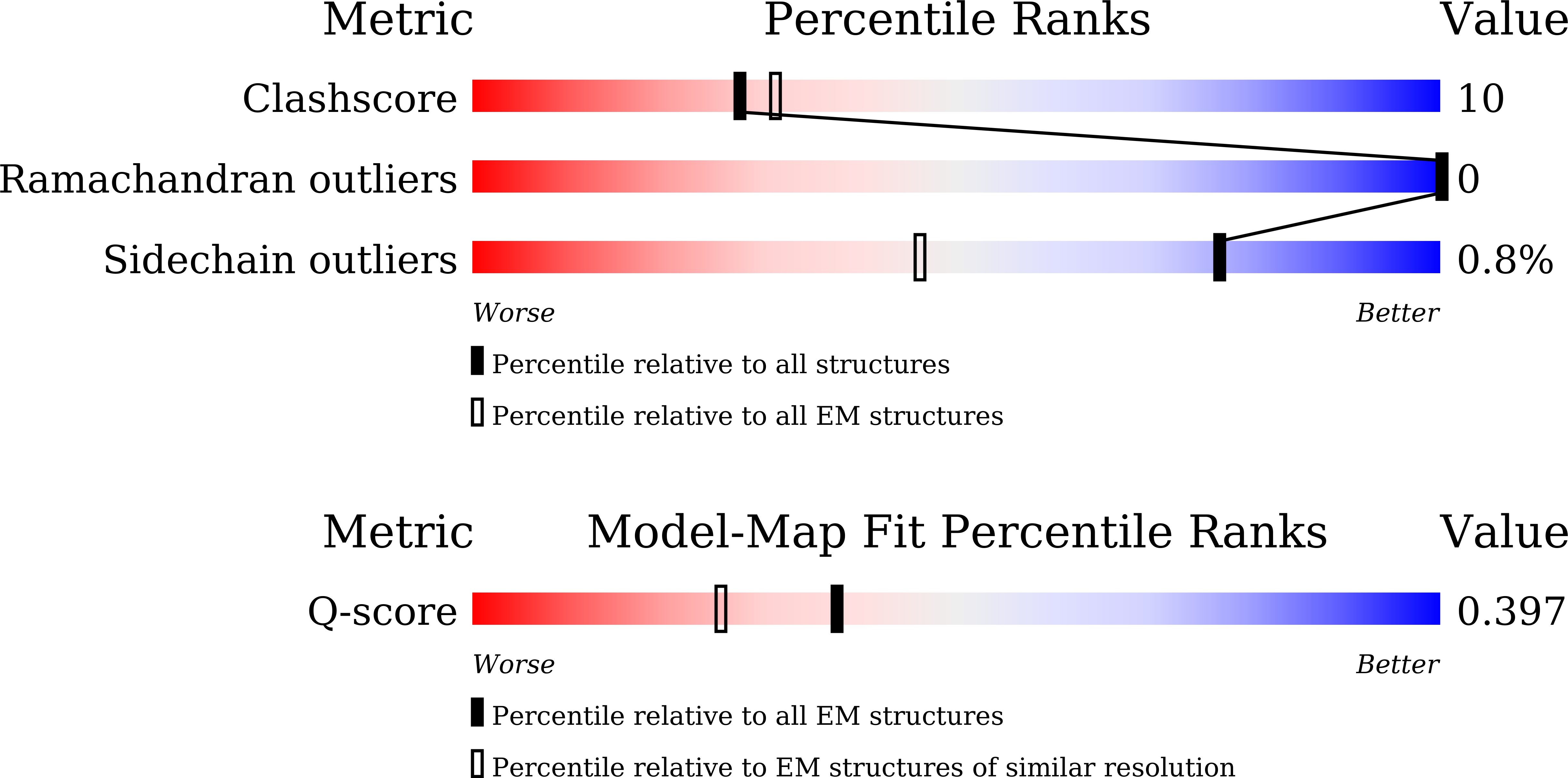
Deposition Date
2024-08-21
Release Date
2025-11-19
Last Version Date
2025-11-19
Entry Detail
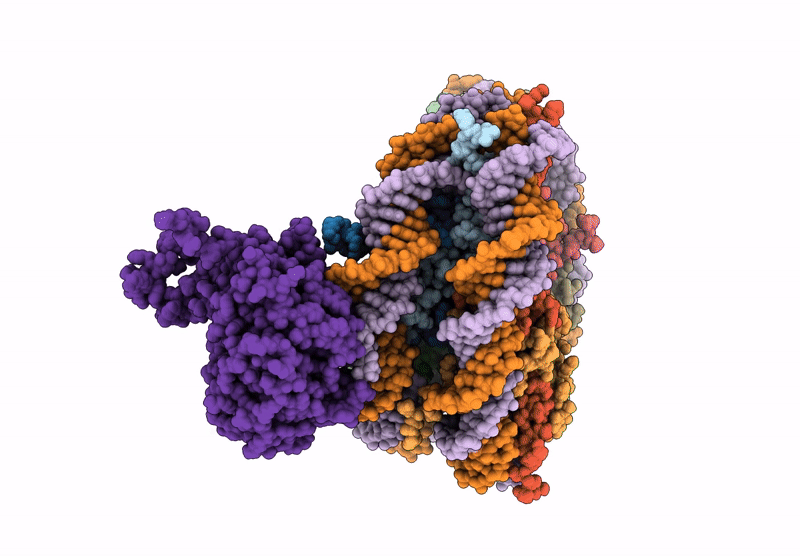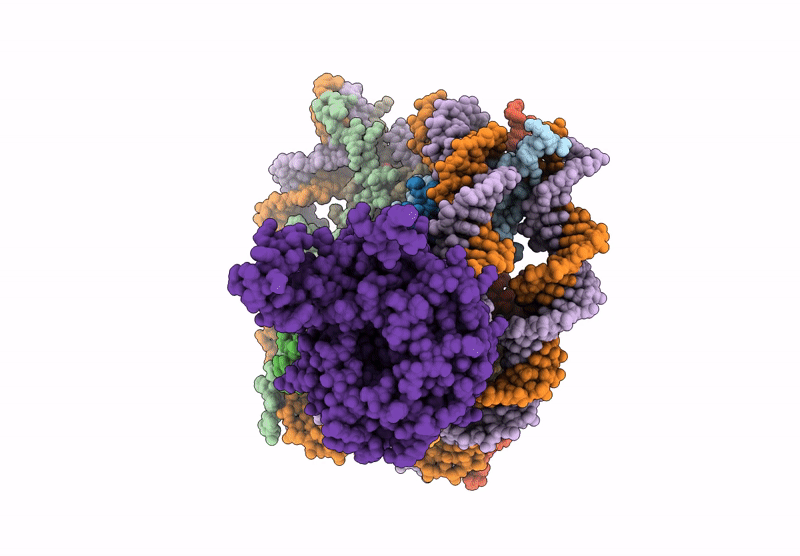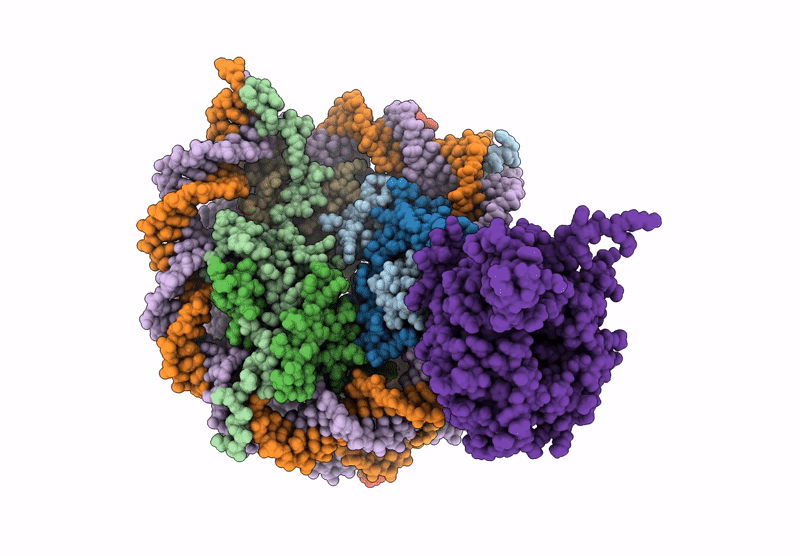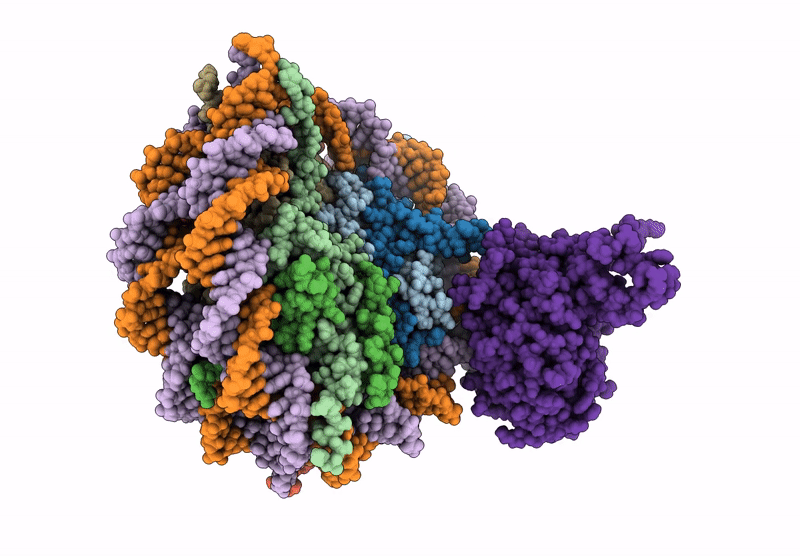
PDB ID:
9J8W
Keywords:
Title:
Cryo-EM structure of NCP-UV-DDB complex containing CPD
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
synthetic construct (Taxon ID: 32630)
synthetic construct (Taxon ID: 32630)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
3.38 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE