Deposition Date
2024-07-26
Release Date
2025-05-07
Last Version Date
2025-05-21
Entry Detail
PDB ID:
9IWX
Keywords:
Title:
Crystal structure of the mouse RIP3 kinase domain(R69H) in complexed with GSK'872
Biological Source:
Source Organism:
Mus musculus (Taxon ID: 10090)
Host Organism:
Method Details:
Experimental Method:
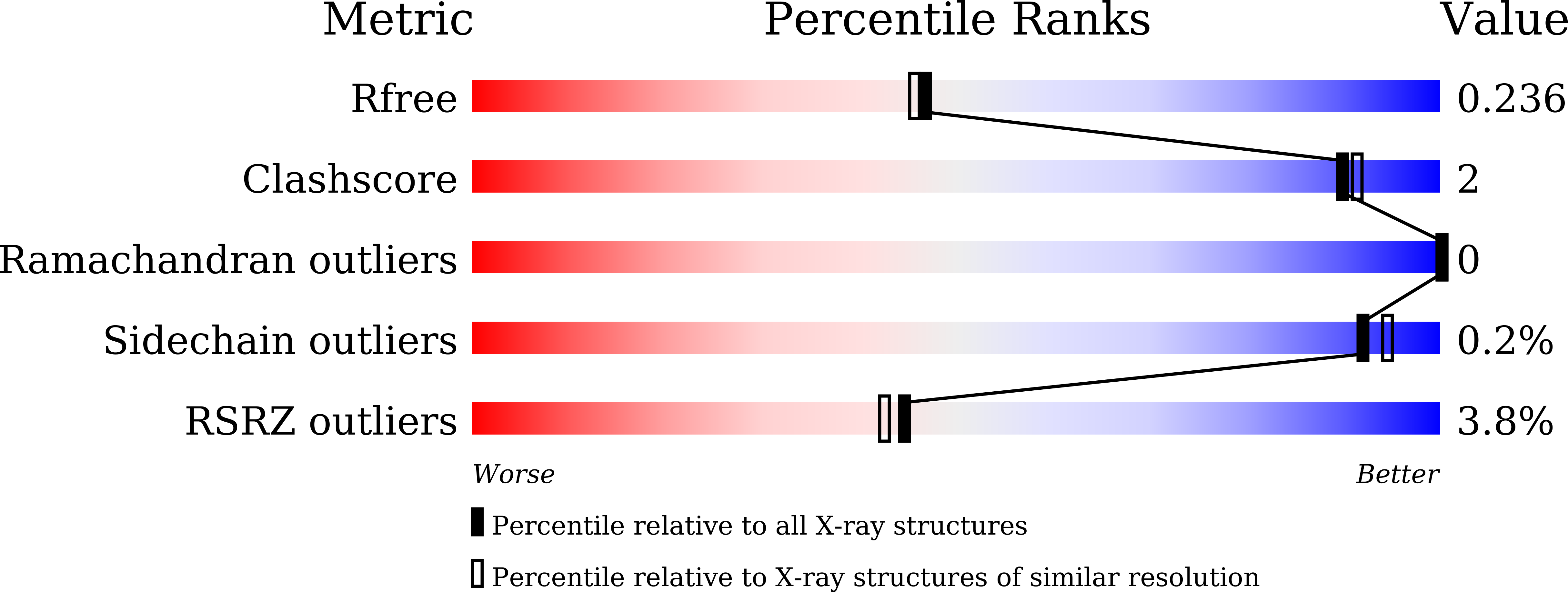
Resolution:
1.99 Å
R-Value Free:
0.23
R-Value Work:
0.18
R-Value Observed:
0.18
Space Group:
C 1 2 1