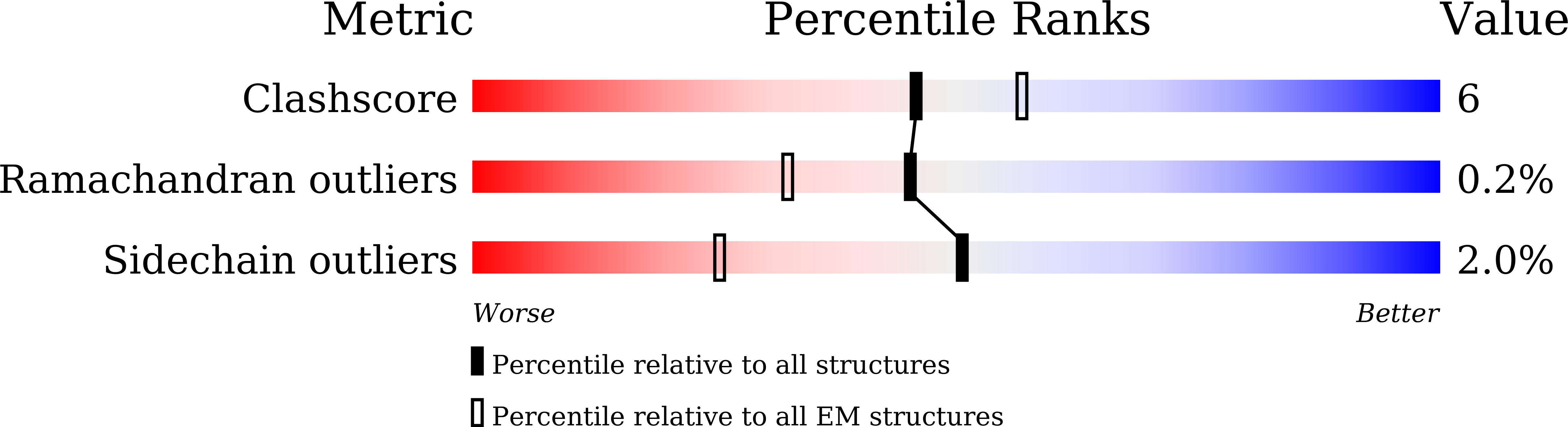
Deposition Date
2024-07-12
Release Date
2025-05-14
Last Version Date
2025-05-14
Entry Detail
PDB ID:
9IQG
Keywords:
Title:
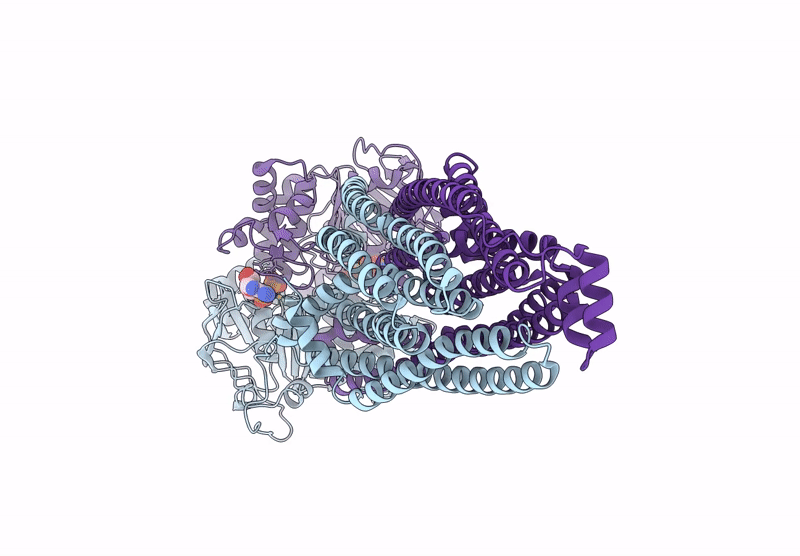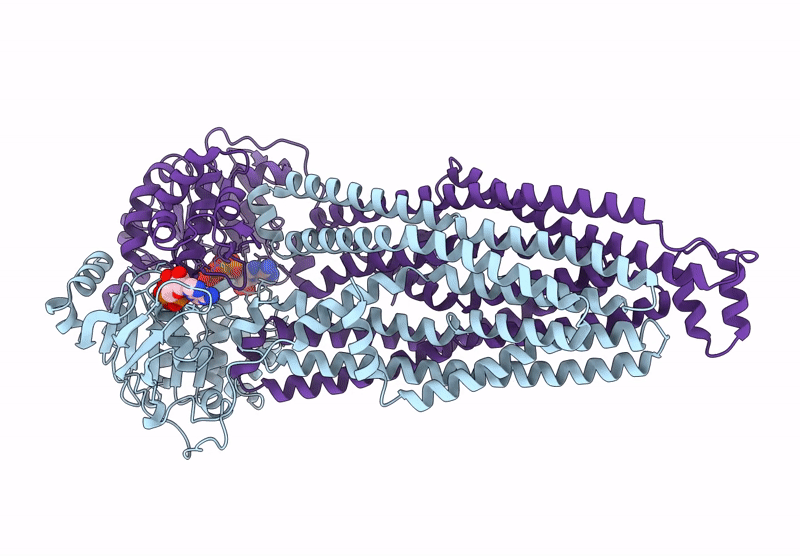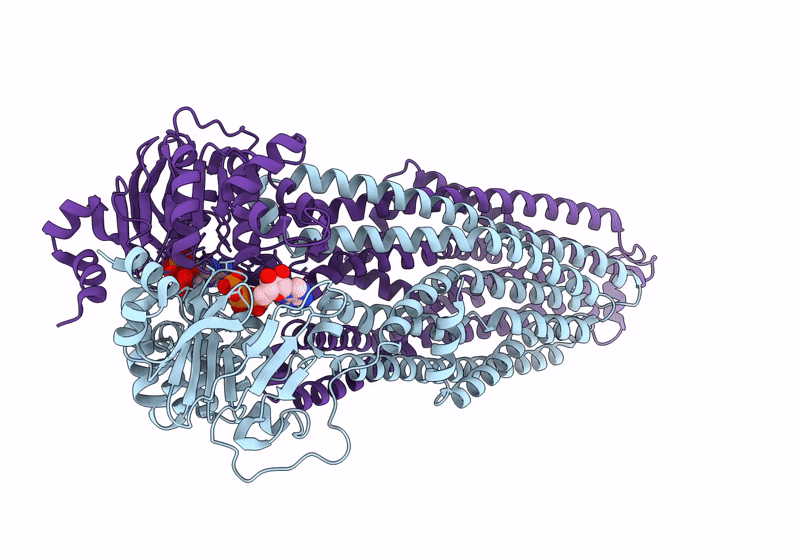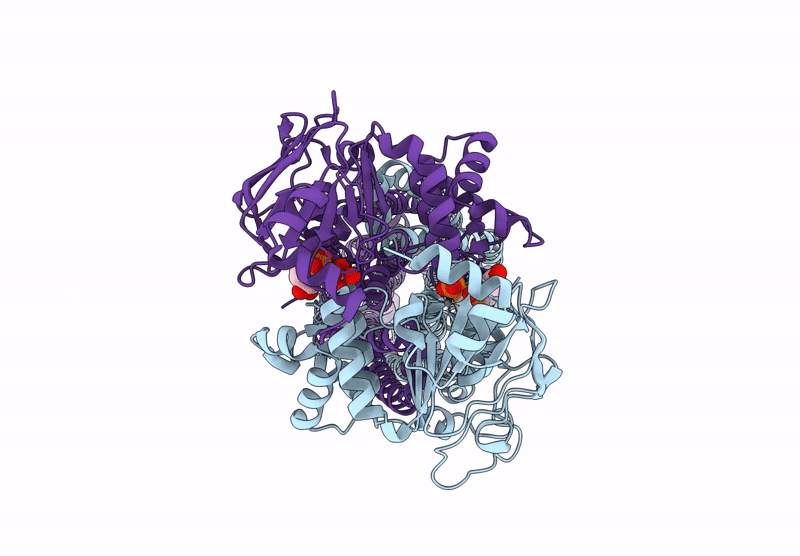
Cryo-EM structure of MsRv1273c/72c from Mycobacterium smegmatis in the ATP|ADP+Vi-bound Occ (Vi) state
Biological Source:
Source Organism(s):
Mycolicibacterium smegmatis MC2 155 (Taxon ID: 246196)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
2.70 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE