Deposition Date
2024-07-08
Release Date
2025-07-16
Last Version Date
2026-01-28
Entry Detail
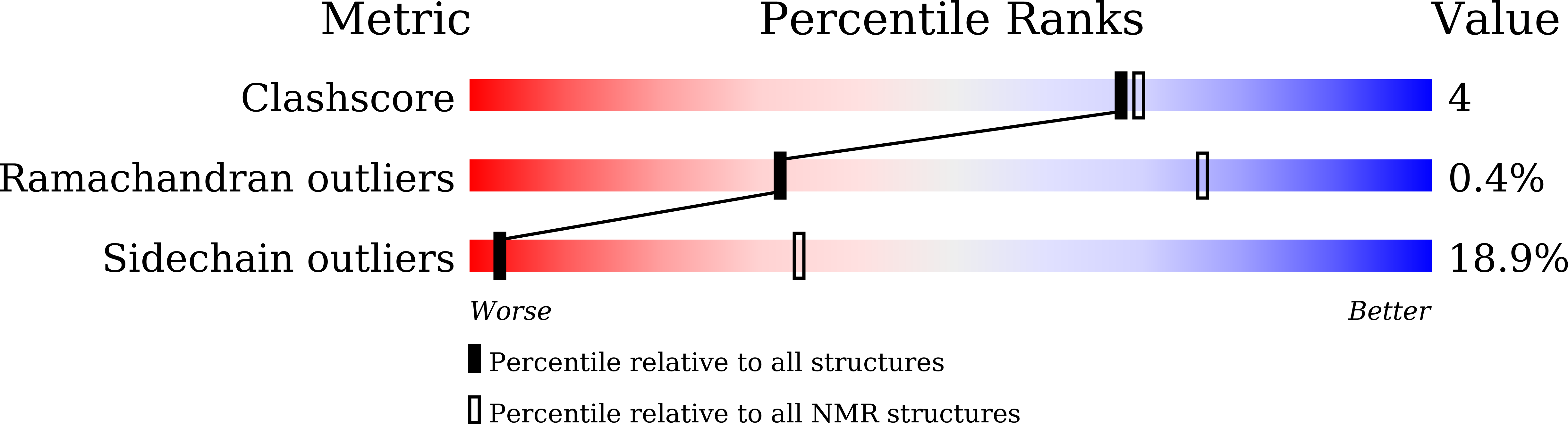
PDB ID:
9IO3
Keywords:
Title:
D-Amino Acid Substituted Antimicrobial Peptides Derived from Tilapia piscidin 4
Biological Source:
Source Organism(s):
Oreochromis niloticus (Taxon ID: 8128)
Method Details:
Experimental Method:
Conformers Calculated:
100
Conformers Submitted:
20
Selection Criteria:
structures with the lowest energy