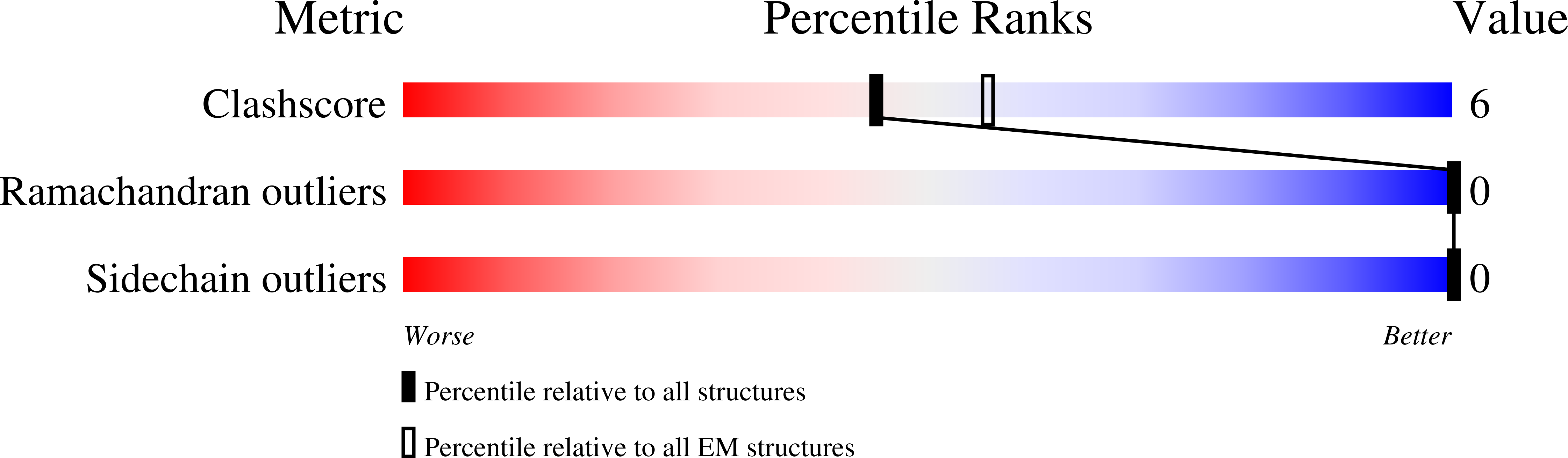
Deposition Date
2025-01-29
Release Date
2025-07-09
Last Version Date
2025-07-30
Entry Detail
PDB ID:
9I6B
Keywords:
Title:
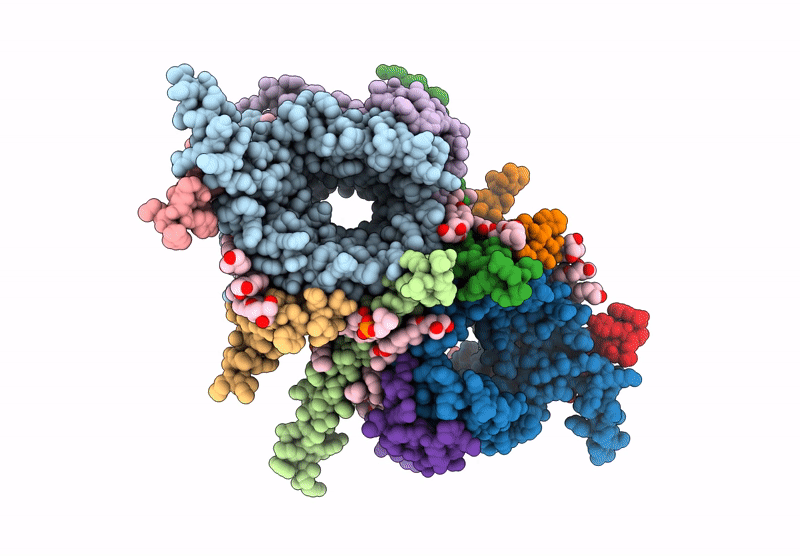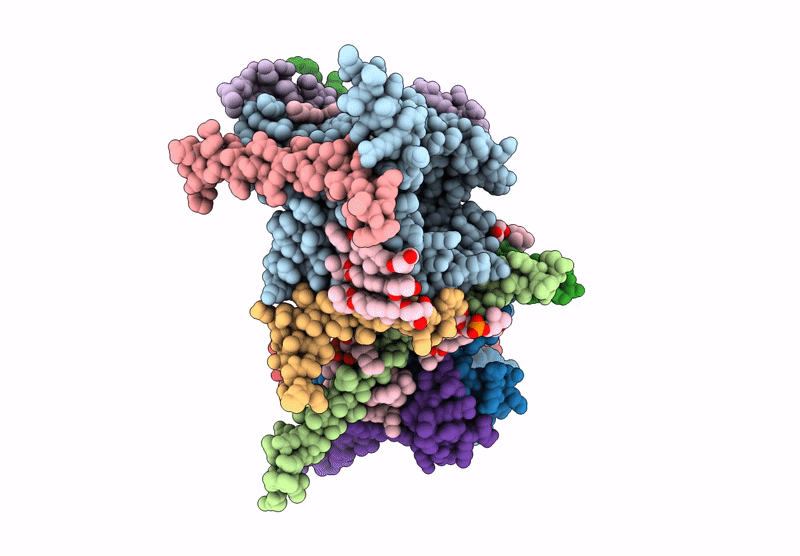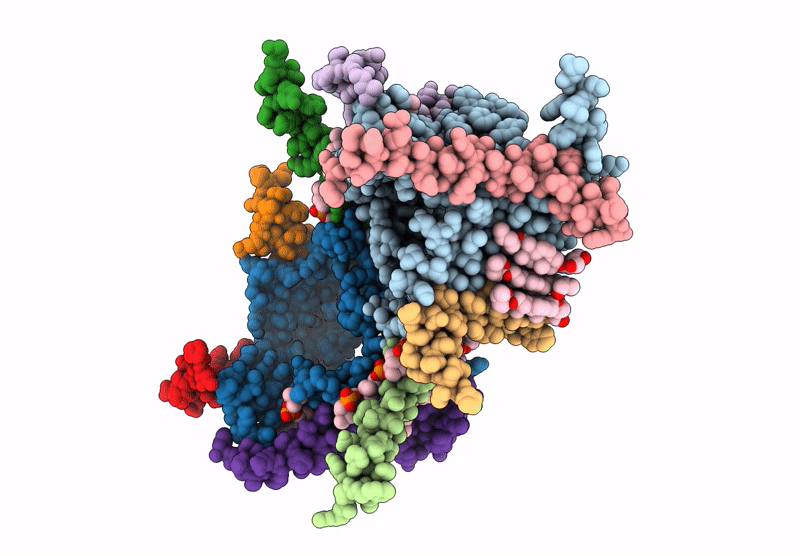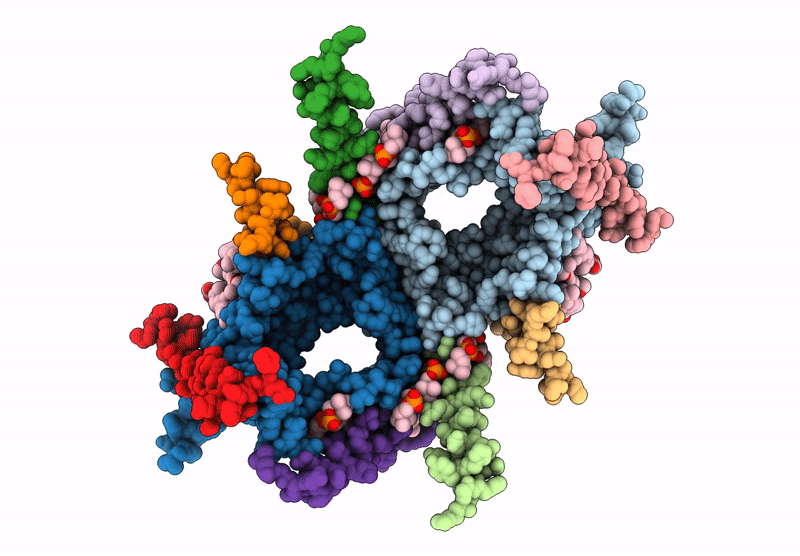
CryoEM structure of the Chaetomium thermophilum TOM core complex at 2.7 angstrom resolution (pALDH treated)
Biological Source:
Source Organism(s):
Thermochaetoides thermophila DSM 1495 (Taxon ID: 759272)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
2.70 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE