Deposition Date
2025-01-09
Release Date
2025-04-30
Last Version Date
2025-04-30
Entry Detail
PDB ID:
9HY2
Keywords:
Title:
sc-4E (scFv derived from the mAb 4E1 against CD93) crystallized at pH 7.5 (monomeric state from gel filtration)
Biological Source:
Source Organism(s):
Mus musculus (Taxon ID: 10090)
Expression System(s):
Method Details:
Experimental Method:
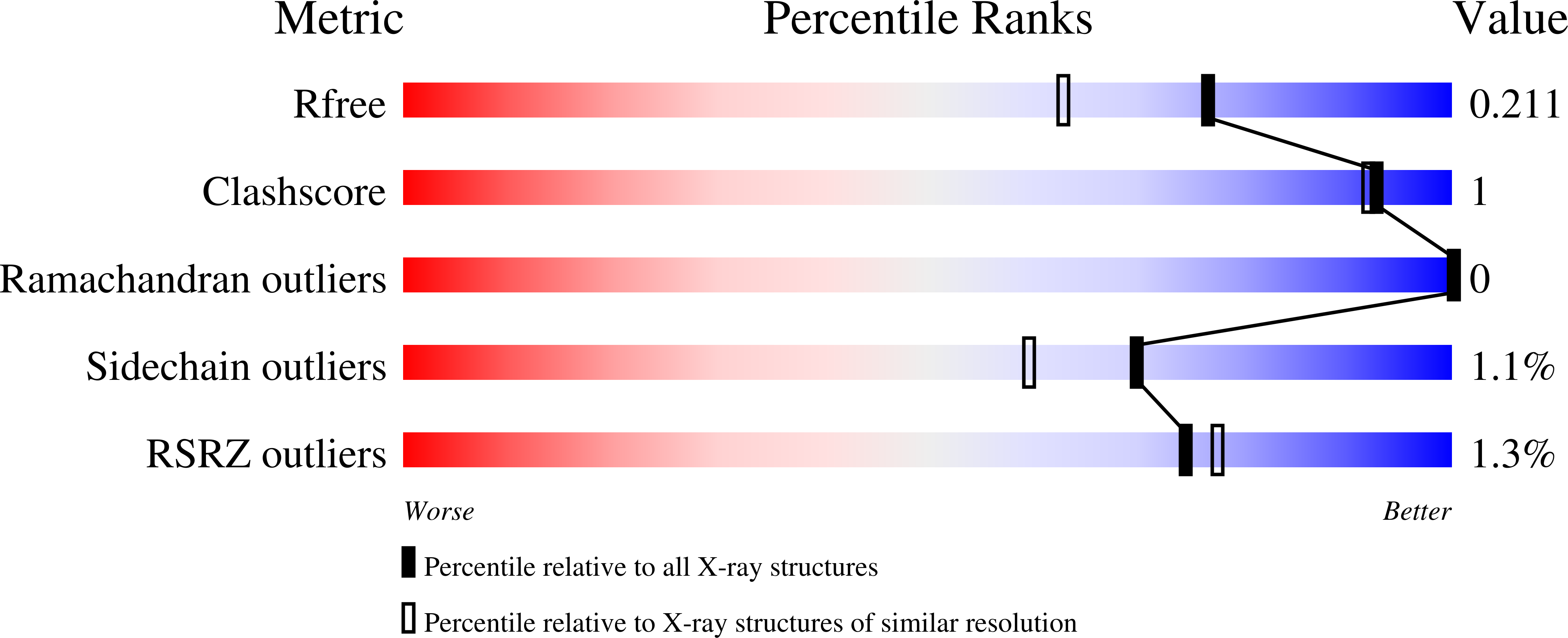
Resolution:
1.70 Å
R-Value Free:
0.21
R-Value Work:
0.17
R-Value Observed:
0.17
Space Group:
P 32 2 1