Deposition Date
2024-12-18
Release Date
2025-04-30
Last Version Date
2025-04-30
Entry Detail
PDB ID:
9HS7
Keywords:
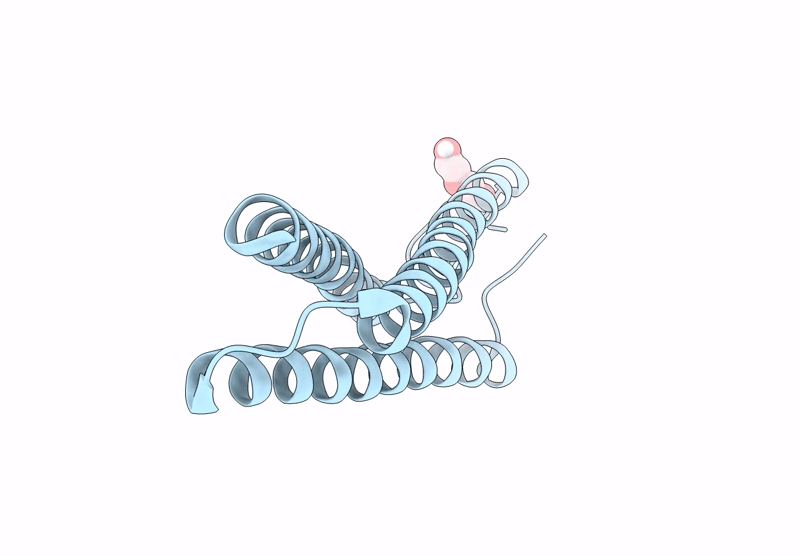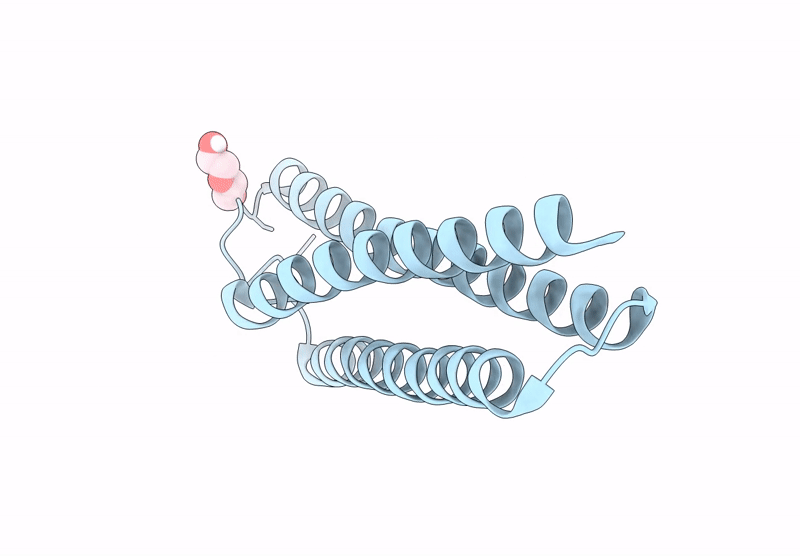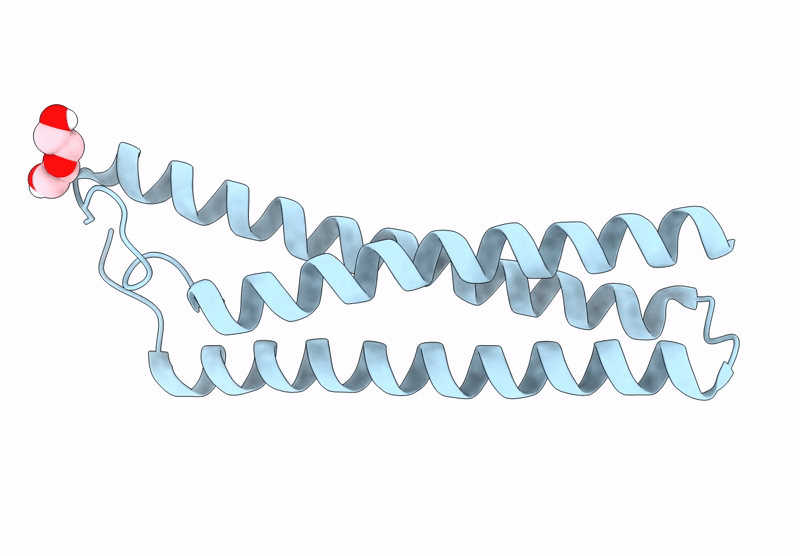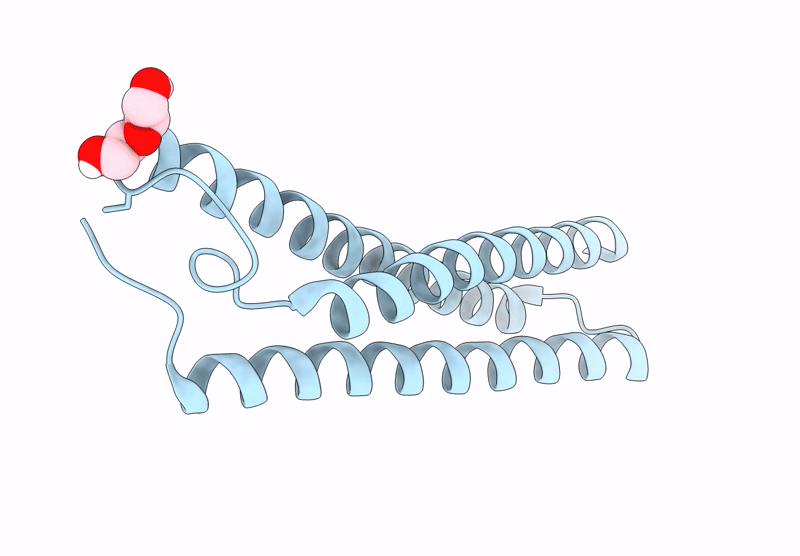
Title:
Anti-HIV-1 chimeric miniprotein mimicking the N-terminal half of gp41 NHR with an extended region targeting the MPER
Biological Source:
Source Organism(s):
Expression System(s):
Method Details:
Experimental Method:
Resolution:
1.70 Å
R-Value Free:
0.23
R-Value Work:
0.20
R-Value Observed:
0.20
Space Group:
P 65


