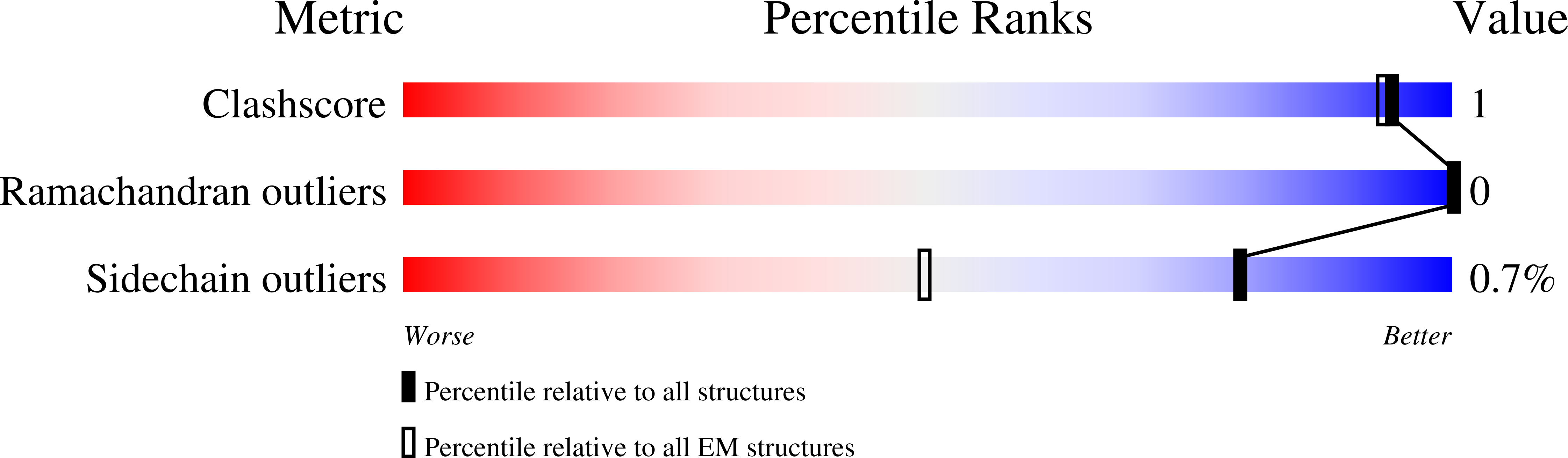
Deposition Date
2024-12-16
Release Date
2025-05-07
Last Version Date
2025-07-30
Entry Detail
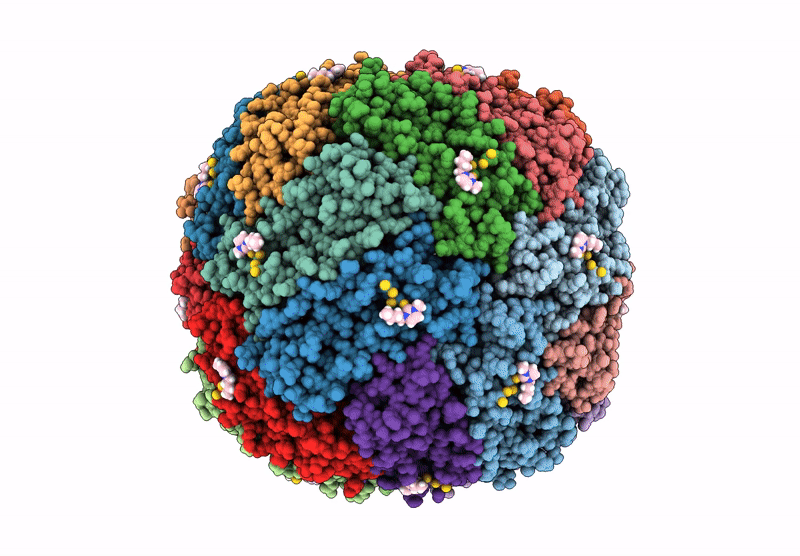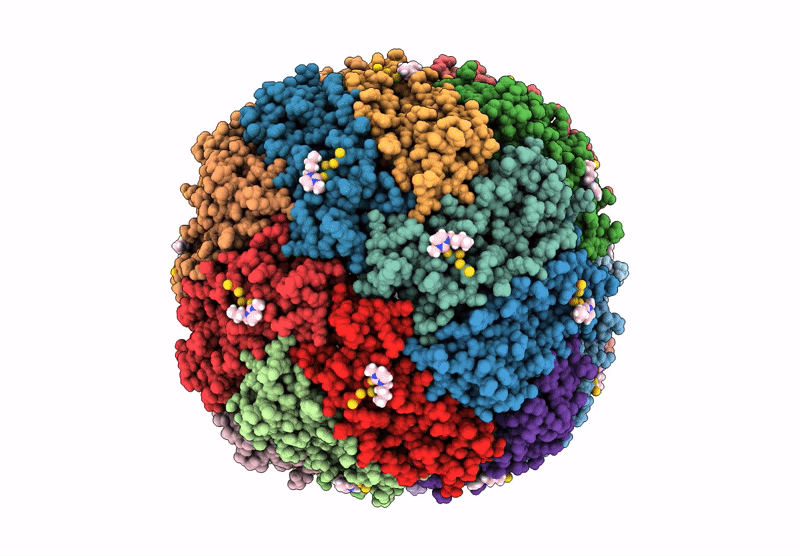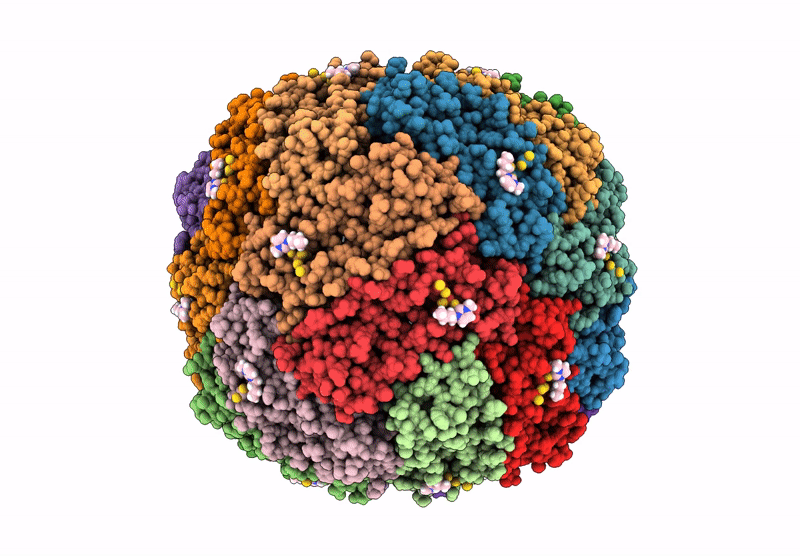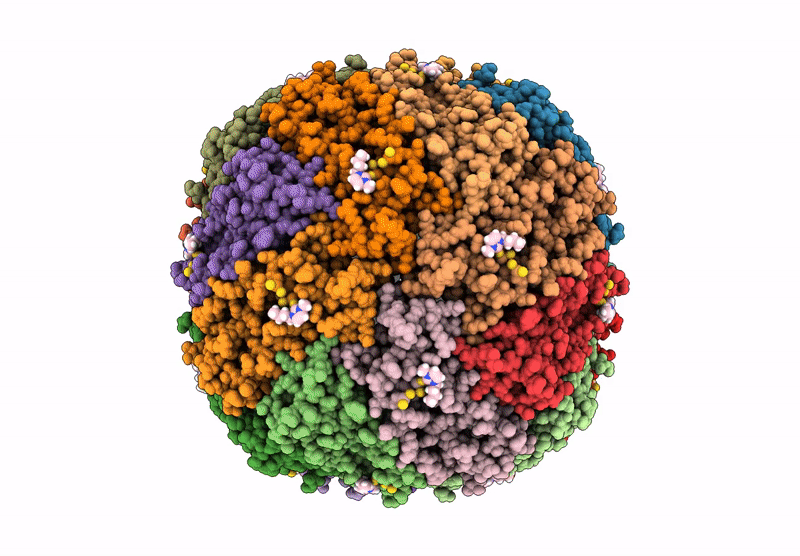
PDB ID:
9HQ6
Keywords:
Title:
Structural insights in the HuHf@gold-monocarbene adduct: aurophilicity revealed in a biological context
Biological Source:
Source Organism:
Homo sapiens (Taxon ID: 9606)
Host Organism:
Method Details:
Experimental Method:
Resolution:
1.51 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE