Deposition Date
2024-12-09
Release Date
2024-12-25
Last Version Date
2025-07-16
Entry Detail
PDB ID:
9HMF
Keywords:
Title:
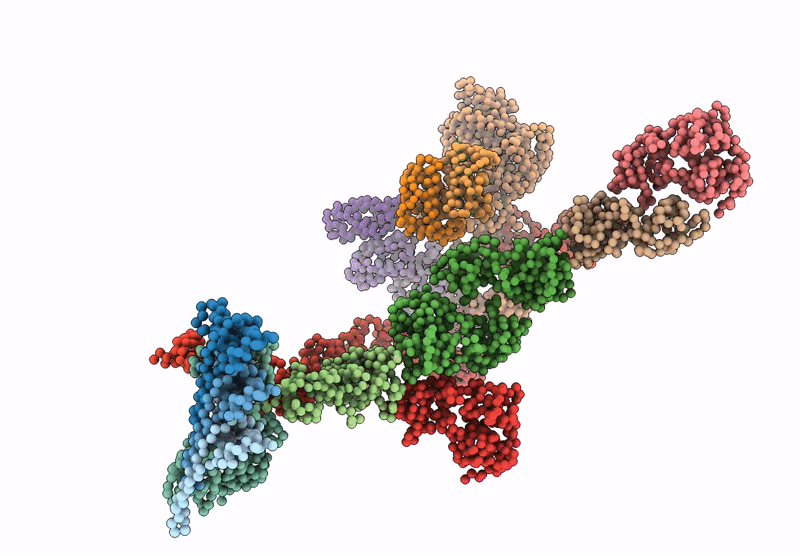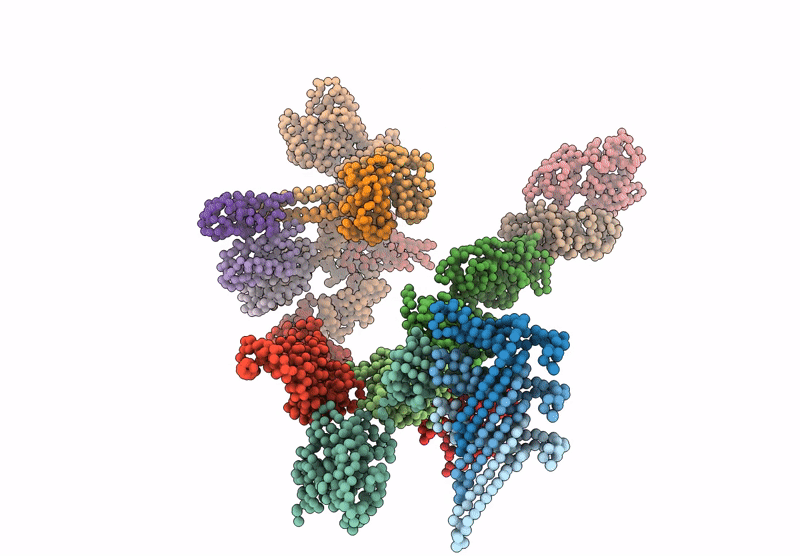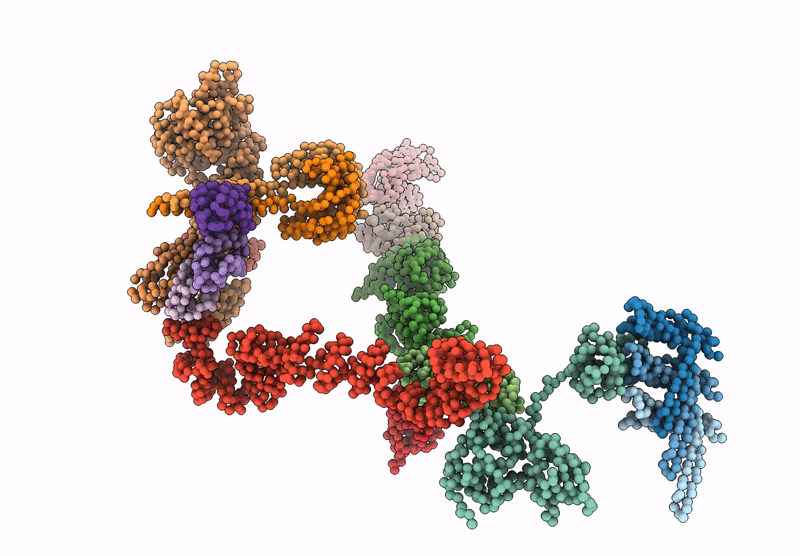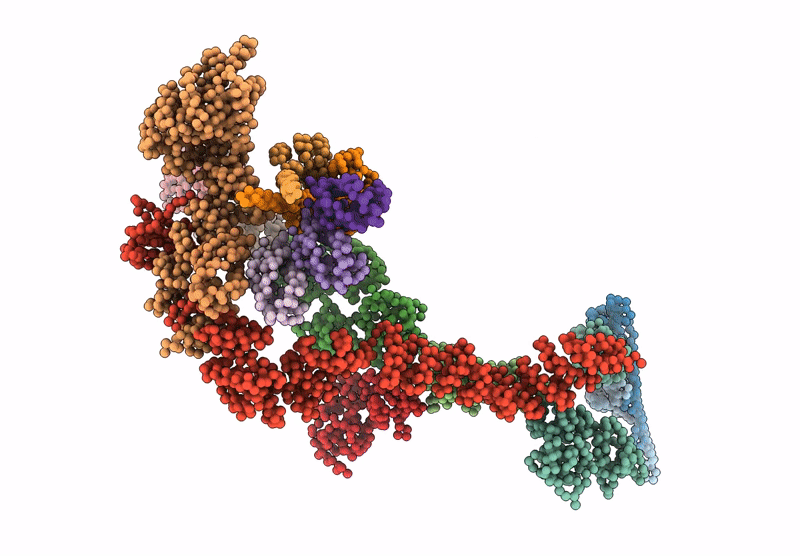
Periplasmic scaffold of the Campylobacter jejuni flagellar motor (alpha carbon trace)
Biological Source:
Source Organism(s):
Campylobacter jejuni (Taxon ID: 197)
Method Details:
Experimental Method:
Resolution:
7.90 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE