Deposition Date
2024-11-01
Release Date
2025-03-05
Last Version Date
2025-05-07
Entry Detail
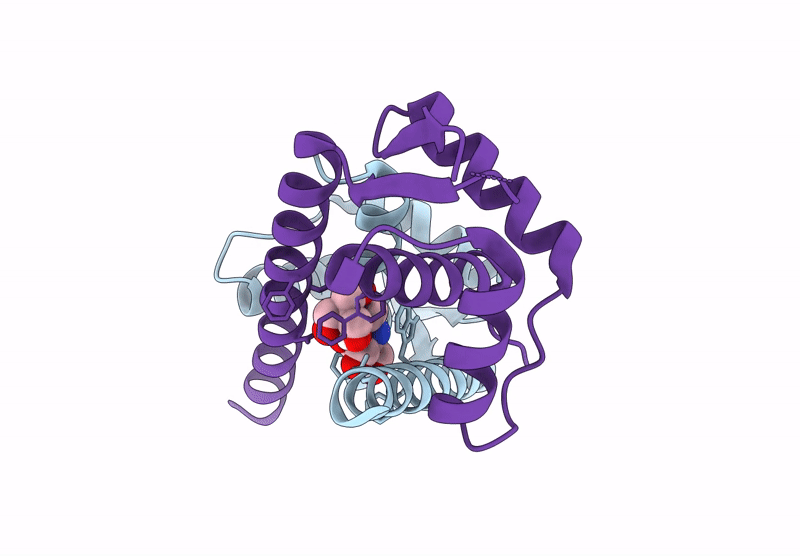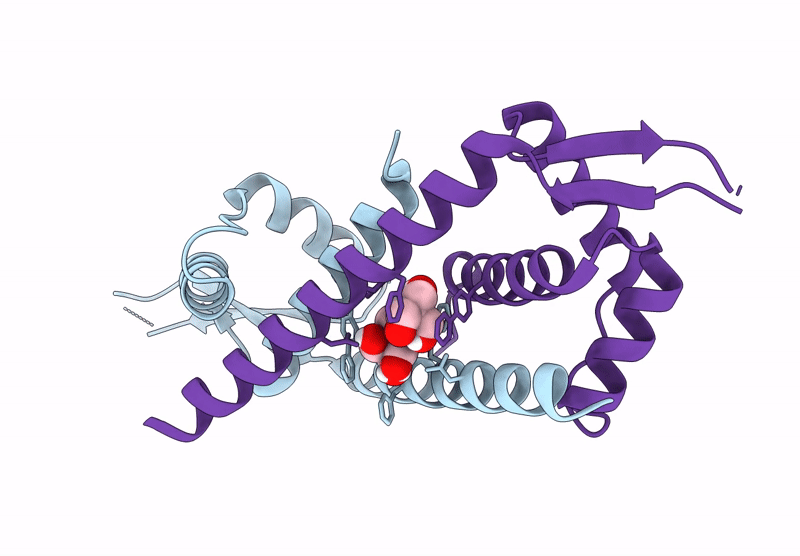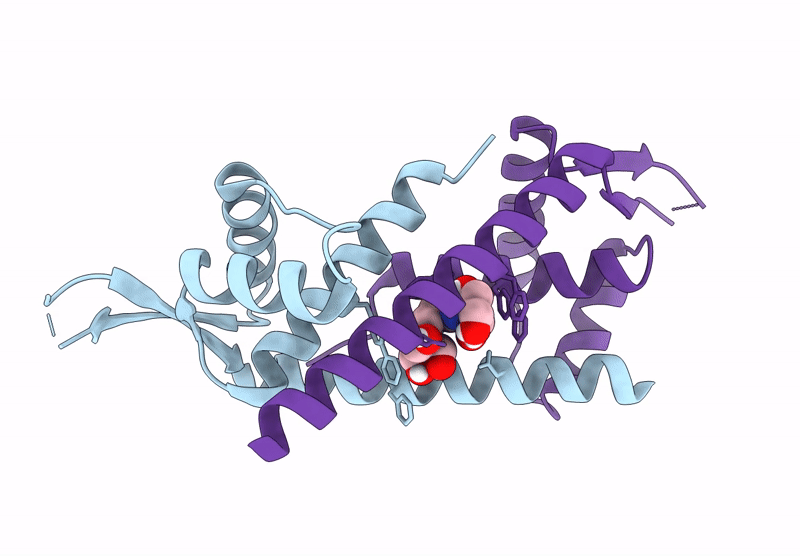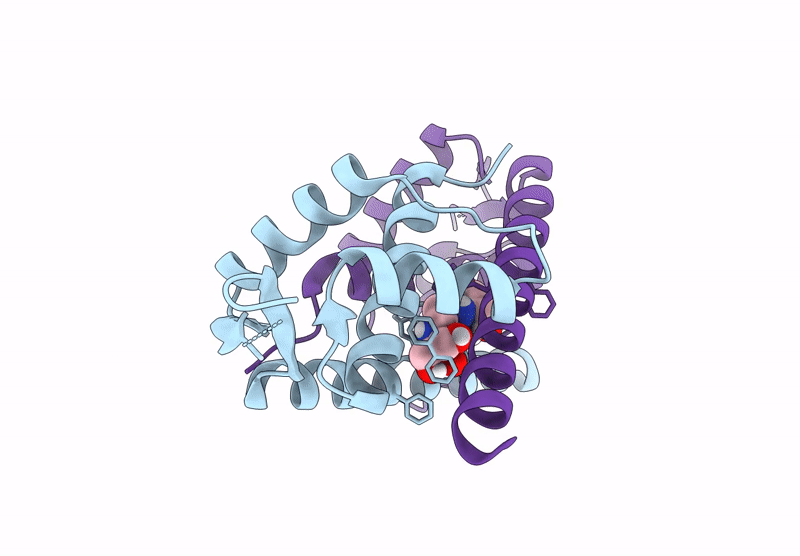
PDB ID:
9H9W
Keywords:
Title:
Crystal structure of metal-free LmrR_V15Bpy in an open state
Biological Source:
Source Organism(s):
Lactococcus cremoris subsp. cremoris MG1363 (Taxon ID: 416870)
Expression System(s):
Method Details:
Experimental Method:
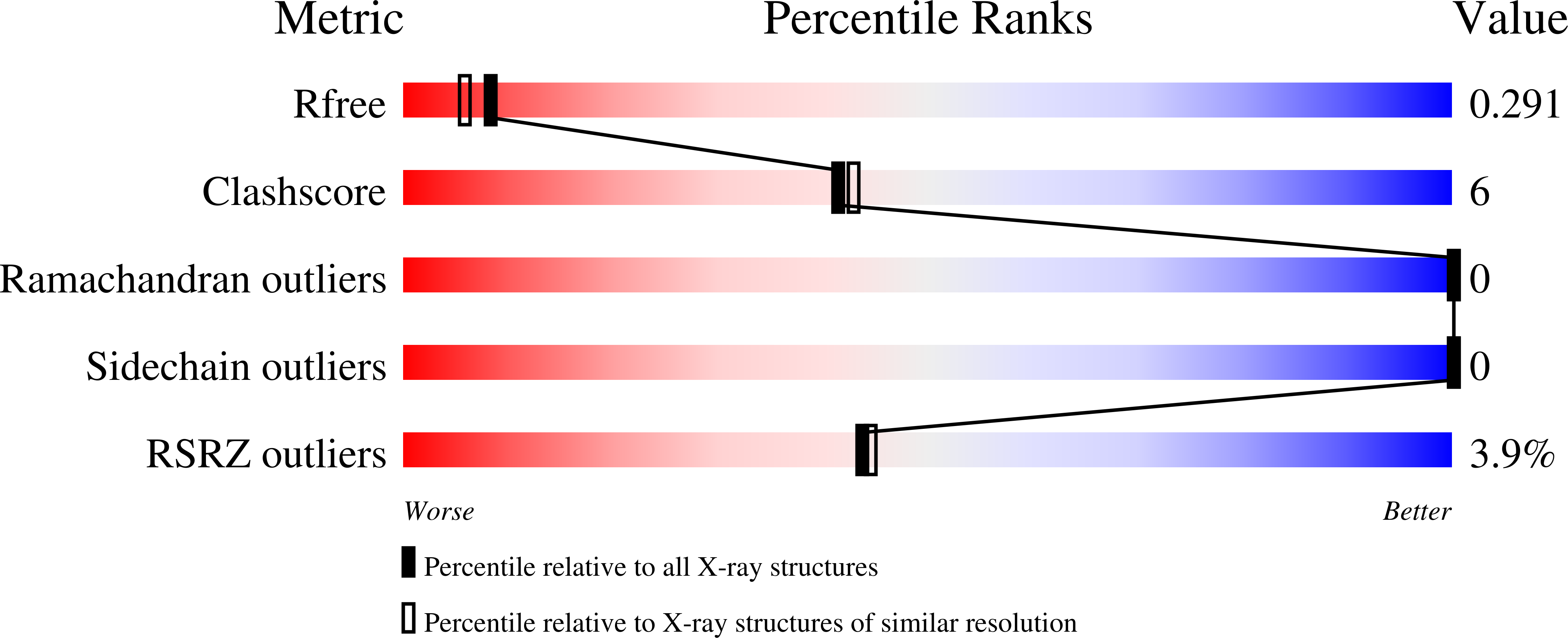
Resolution:
2.26 Å
R-Value Free:
0.29
R-Value Work:
0.22
R-Value Observed:
0.22
Space Group:
C 1 2 1