Deposition Date
2024-10-28
Release Date
2025-04-30
Last Version Date
2025-06-04
Entry Detail
PDB ID:
9H87
Keywords:
Title:
Crystal structure of LmrR variant V15aY with Val15 replaced by 3-aminotyrosine
Biological Source:
Source Organism:
Lactococcus cremoris subsp. cremoris MG1363 (Taxon ID: 416870)
Host Organism:
Method Details:
Experimental Method:
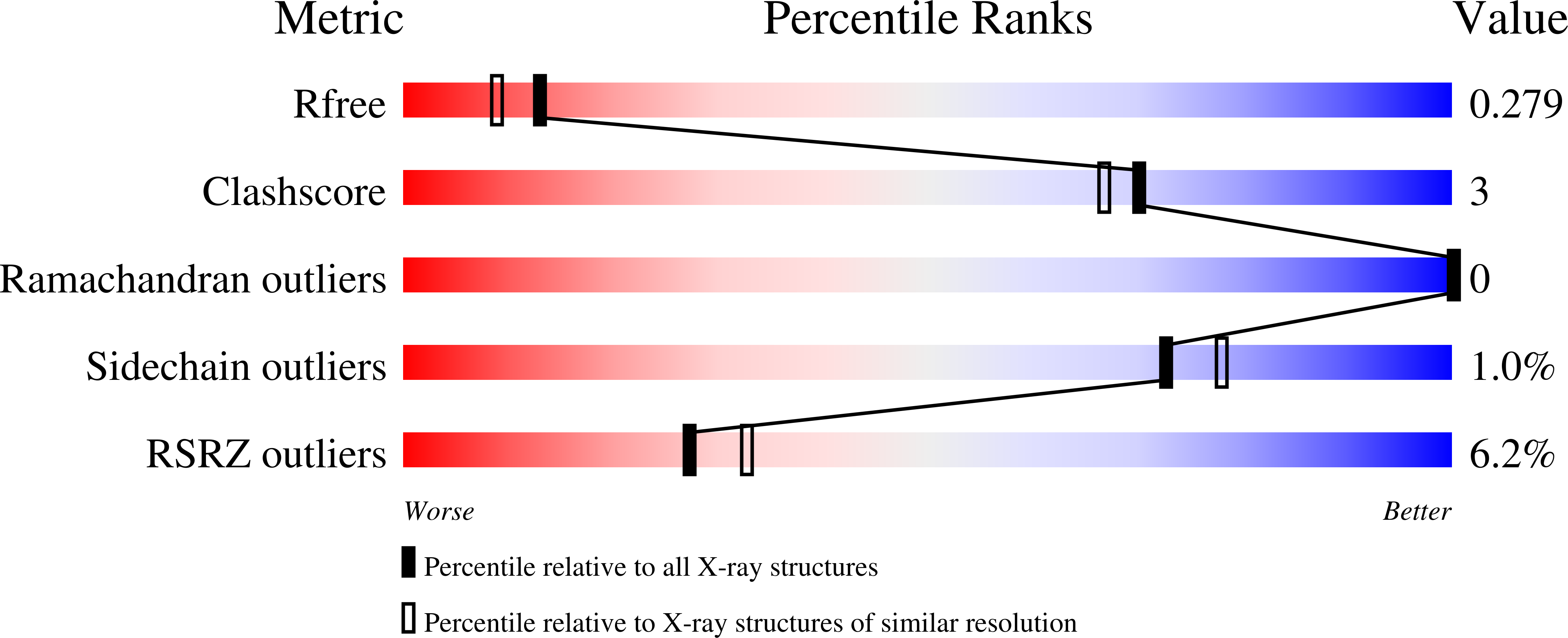
Resolution:
2.15 Å
R-Value Free:
0.26
R-Value Work:
0.22
R-Value Observed:
0.22
Space Group:
P 32 1 2