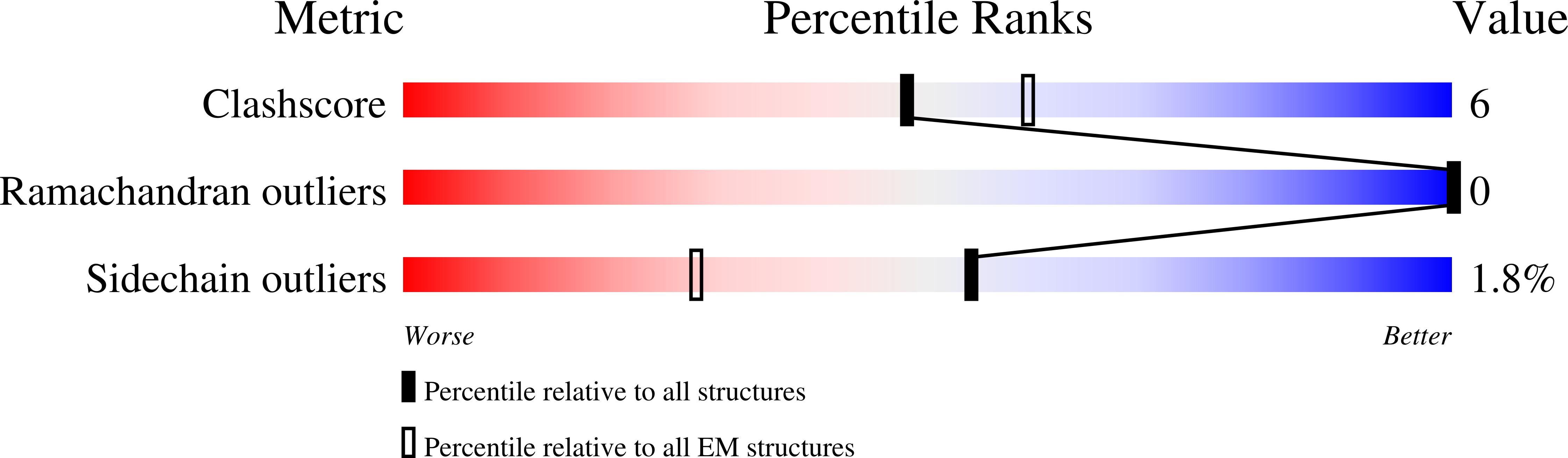Deposition Date
2024-10-26
Release Date
2025-01-01
Last Version Date
2025-07-02
Entry Detail
PDB ID:
9H76
Keywords:
Title:
Bacterial LAT transporter BASC in complex with L-Ala and NB53
Biological Source:
Source Organism:
Carnobacterium sp. AT7 (Taxon ID: 333990)
Lama glama (Taxon ID: 9844)
Lama glama (Taxon ID: 9844)
Host Organism:
Method Details:
Experimental Method:
Resolution:
2.90 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE