Deposition Date
2024-10-18
Release Date
2025-08-27
Last Version Date
2025-08-27
Entry Detail
PDB ID:
9H4F
Keywords:
Title:
Structure of Imine Reductase 361 from Micromonospora sp. mutant M125W/I127F/L179V/H250L
Biological Source:
Source Organism:
Micromonospora (Taxon ID: 1873)
Host Organism:
Method Details:
Experimental Method:
Resolution:
2.77 Å
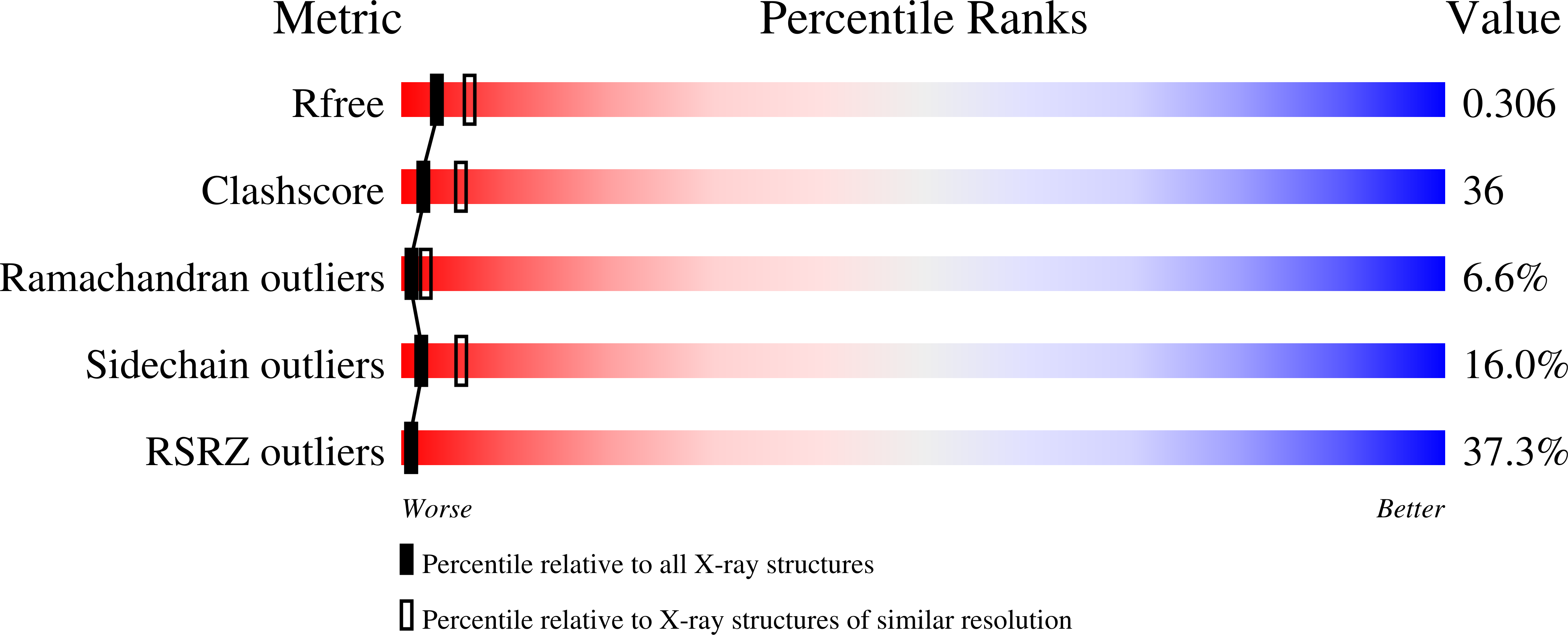
R-Value Free:
0.30
R-Value Work:
0.22
R-Value Observed:
0.22
Space Group:
I 21 3