Deposition Date
2024-10-04
Release Date
2025-09-10
Last Version Date
2025-11-26
Entry Detail
PDB ID:
9GZQ
Keywords:
Title:
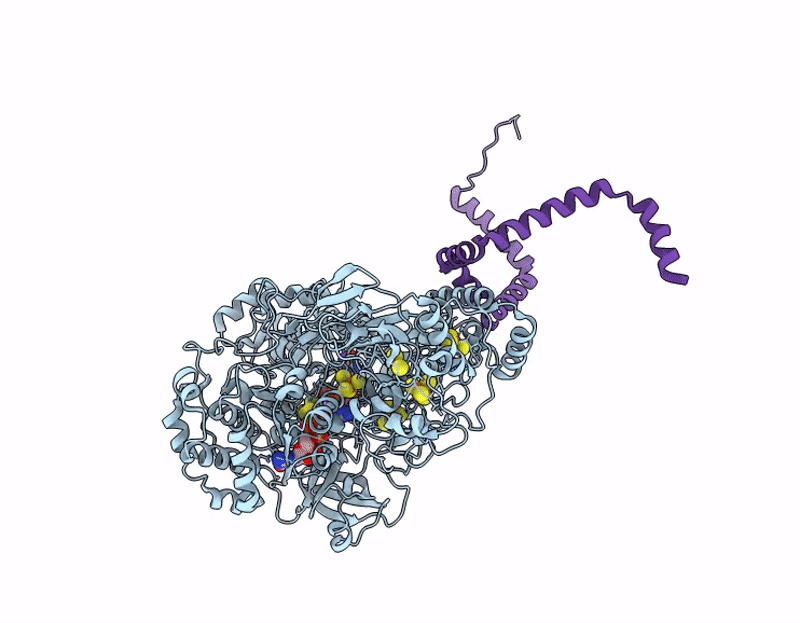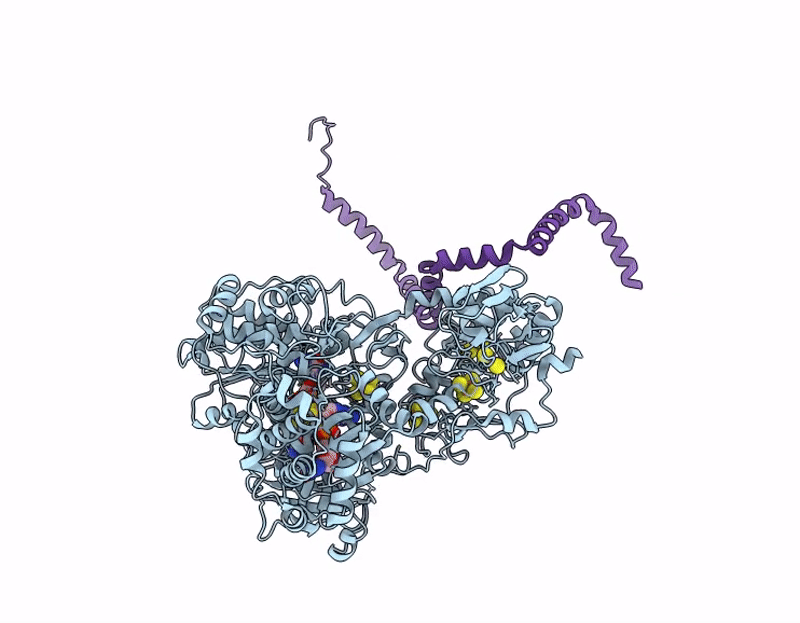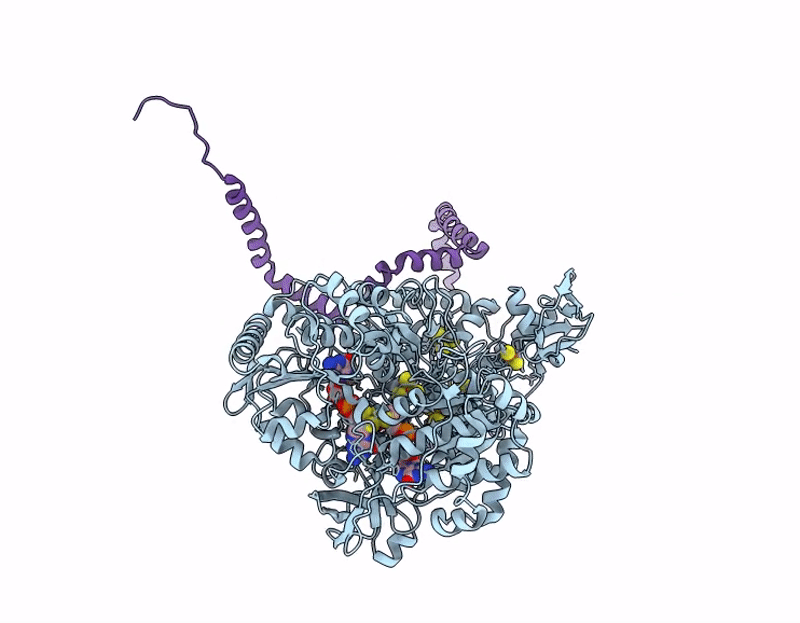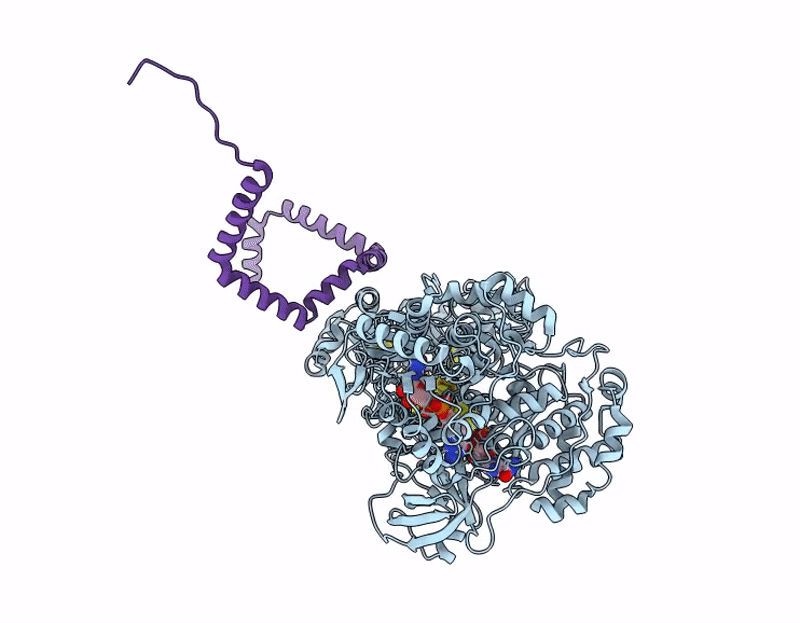
Structure of ForCE lacking the Helical Membrane Plug-in (HMP; DUF1641)
Biological Source:
Source Organism:
Bacillus subtilis (Taxon ID: 1423)
Host Organism:
Method Details:
Experimental Method:
Resolution:
3.63 Å
R-Value Free:
0.31
R-Value Work:
0.28
R-Value Observed:
0.28
Space Group:
I 4 2 2