Deposition Date
2024-08-30
Release Date
2024-12-25
Last Version Date
2025-01-29
Entry Detail
PDB ID:
9GN4
Keywords:
Title:
Nucleoside-2'-deoxyribosyltransferase from Lactobacillus leichmannii. Y7F/D72N mutant with cytidine
Biological Source:
Source Organism:
Lactobacillus leichmannii (Taxon ID: 28039)
Host Organism:
Method Details:
Experimental Method:
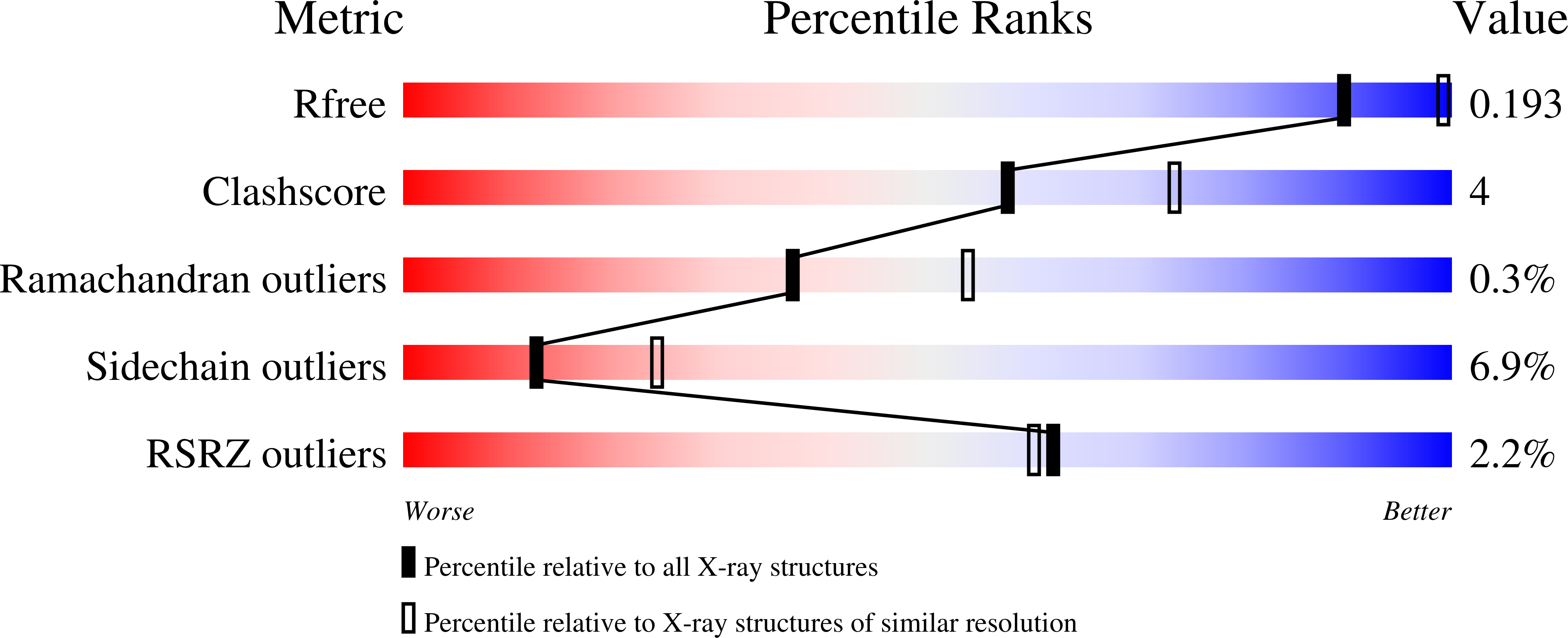
Resolution:
2.48 Å
R-Value Free:
0.18
R-Value Work:
0.16
R-Value Observed:
0.16
Space Group:
I 21 3