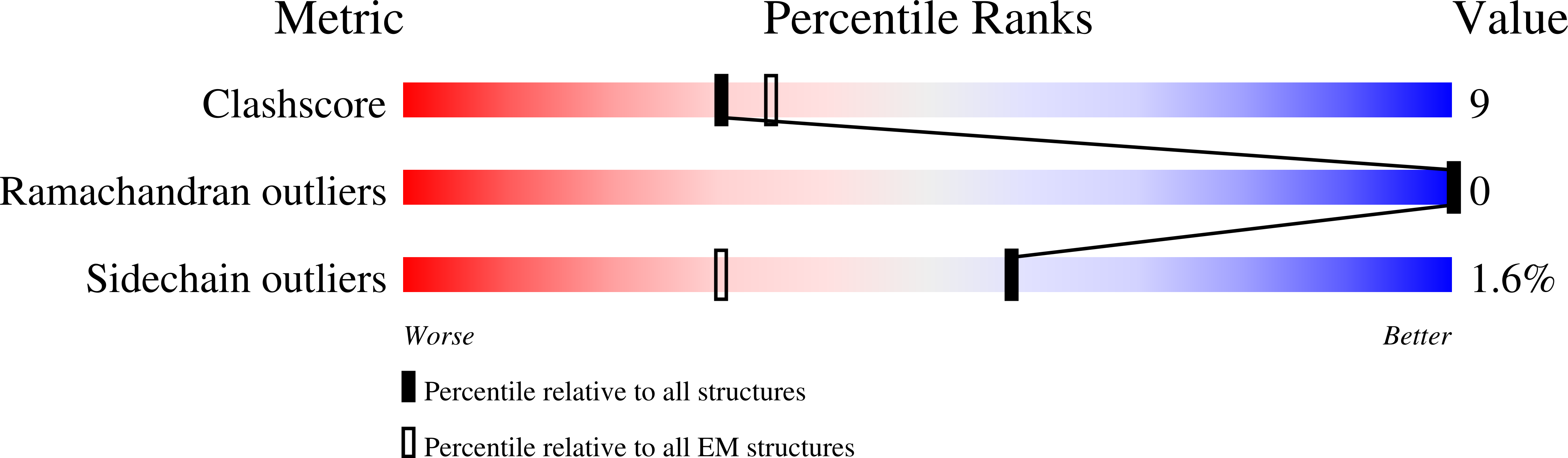
Deposition Date
2024-08-30
Release Date
2025-04-02
Last Version Date
2025-07-09
Entry Detail
PDB ID:
9GMZ
Keywords:
Title:
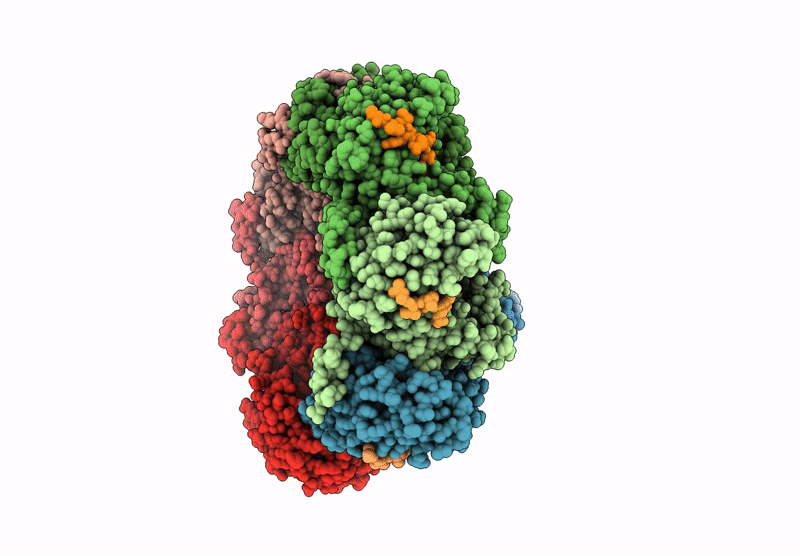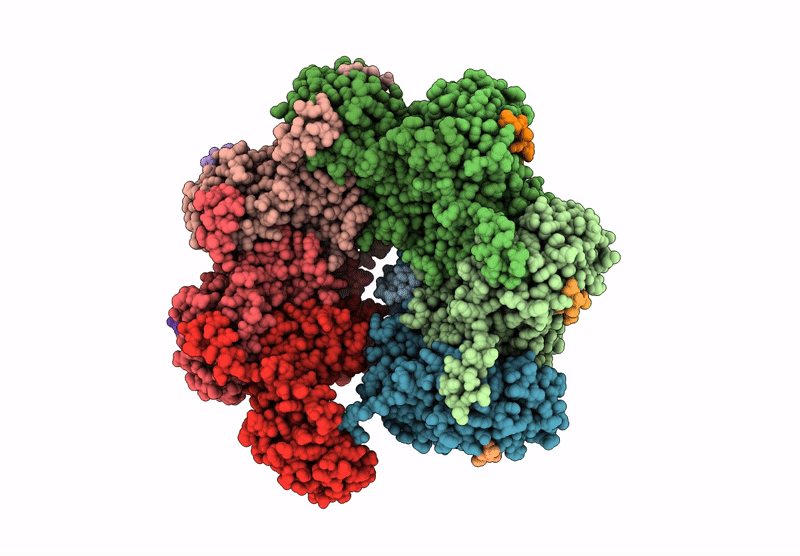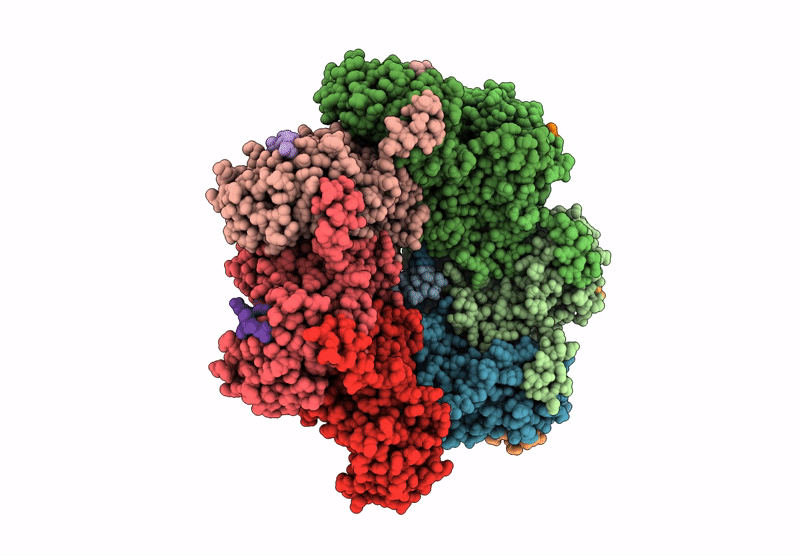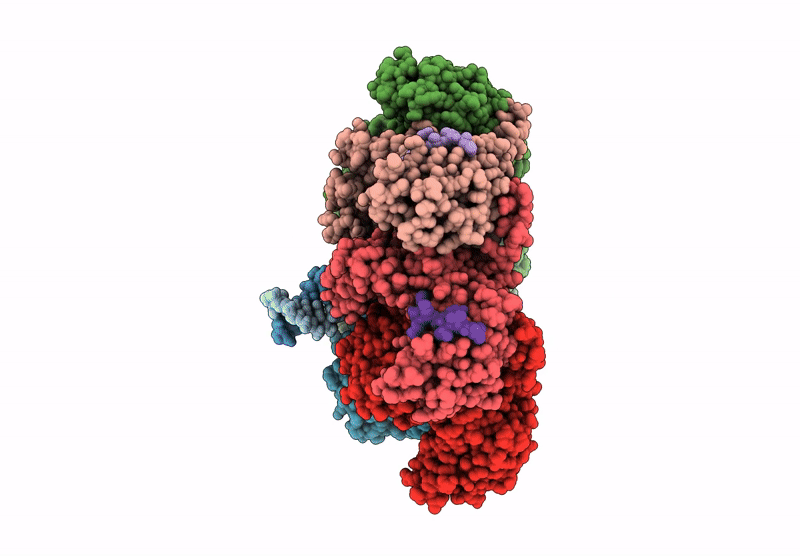
CryoEM structure of PmcTnsC-dsDNA-AMPPNP in complex with PmcTnsAB hook
Biological Source:
Source Organism:
Peltigera membranacea (Taxon ID: 161997)
Escherichia coli (Taxon ID: 562)
Escherichia coli (Taxon ID: 562)
Host Organism:
Method Details:
Experimental Method:
Resolution:
3.20 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE