Deposition Date
2024-08-28
Release Date
2025-06-25
Last Version Date
2025-06-25
Entry Detail
PDB ID:
9GM3
Keywords:
Title:
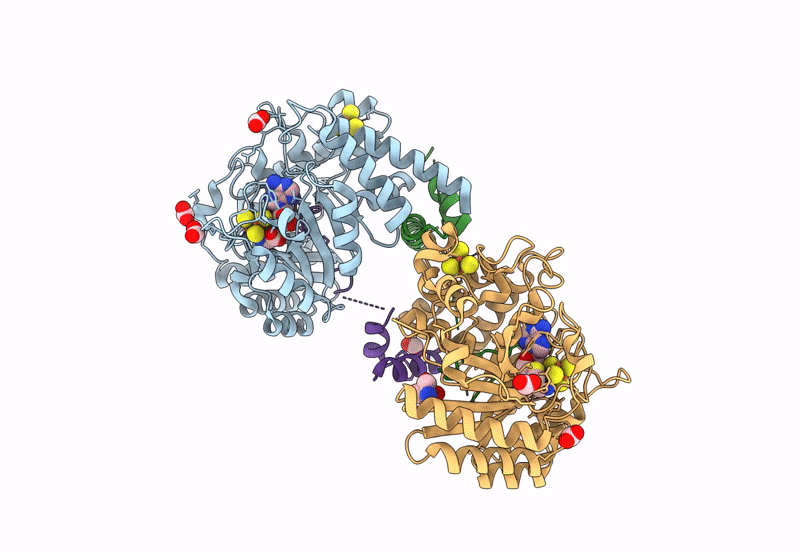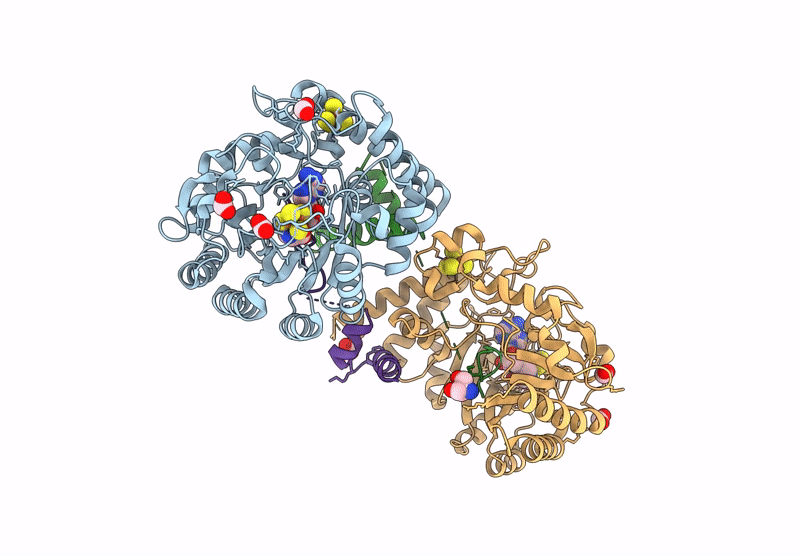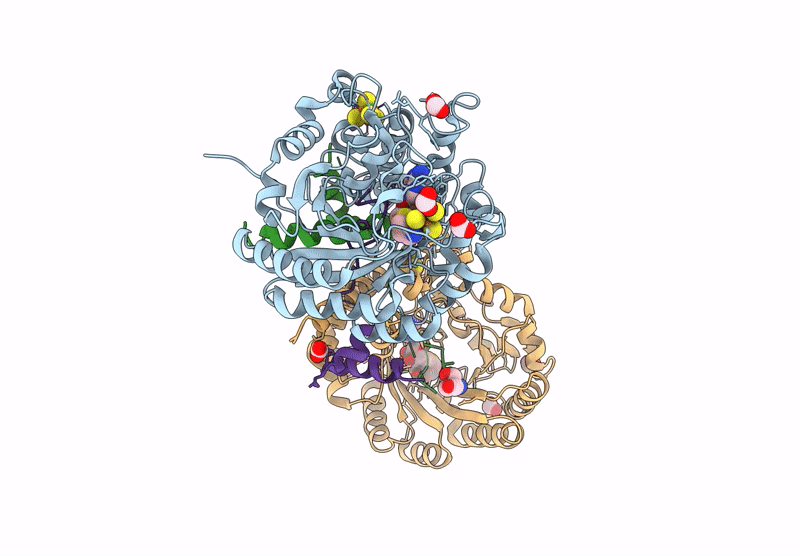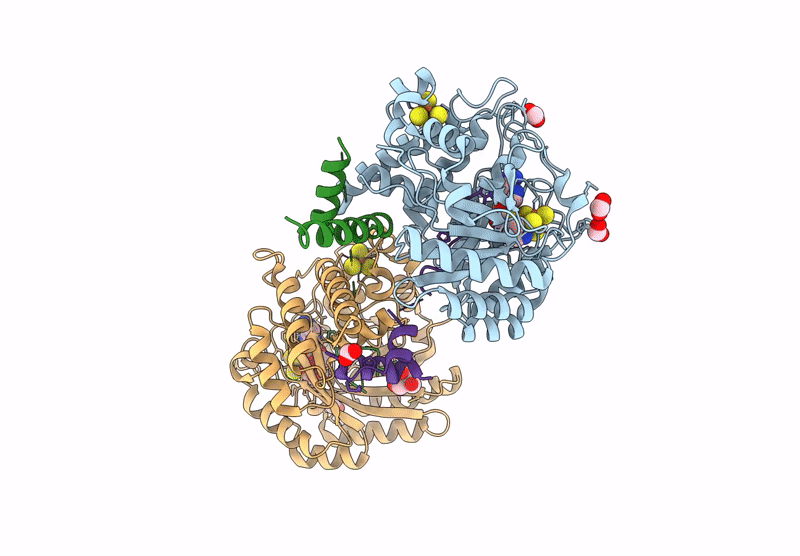
Crystal structure of the complex formed between the radical SAM protein ChlB and the leader region of its precursor substrate ChlA
Biological Source:
Source Organism:
Fischerella (Taxon ID: 1190)
Host Organism:
Method Details:
Experimental Method:
Resolution:
1.65 Å
R-Value Free:
0.21
R-Value Work:
0.19
R-Value Observed:
0.19
Space Group:
C 1 2 1


