Deposition Date
2024-08-23
Release Date
2024-10-02
Last Version Date
2024-10-02
Entry Detail
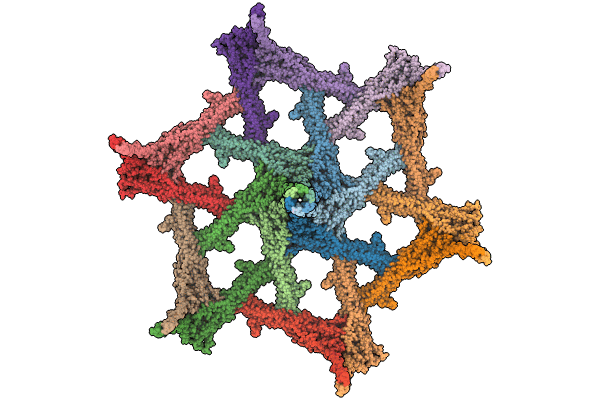
PDB ID:
9GK2
Keywords:
Title:
Surface-layer (S-layer) PS2 protein from Corynebacterium glutamicum
Biological Source:
Source Organism(s):
Corynebacterium glutamicum (Taxon ID: 1718)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
2.50 Å
Aggregation State:
2D ARRAY
Reconstruction Method:
SINGLE PARTICLE


