Deposition Date
2024-07-23
Release Date
2024-11-27
Last Version Date
2024-12-11
Entry Detail
PDB ID:
9G8C
Keywords:
Title:
Crystal structure of the photosensory core module (PCM) of a cyano-phenylalanine mutant oCNF165 of the bathy phytochrome Agp2 from Agrobacterium fabrum in the Pfr state.
Biological Source:
Source Organism(s):
Agrobacterium fabrum str. C58 (Taxon ID: 176299)
Expression System(s):
Method Details:
Experimental Method:
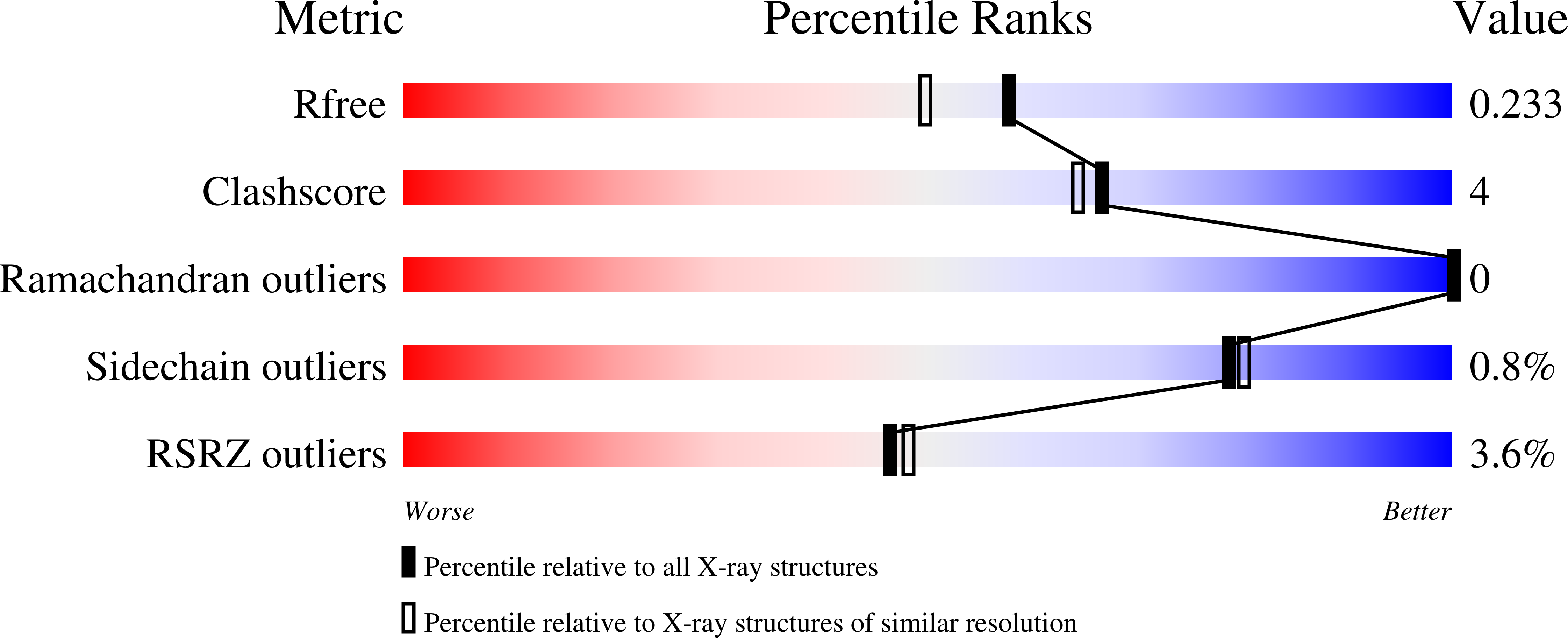
Resolution:
1.90 Å
R-Value Free:
0.22
R-Value Work:
0.18
R-Value Observed:
0.18
Space Group:
P 21 21 21