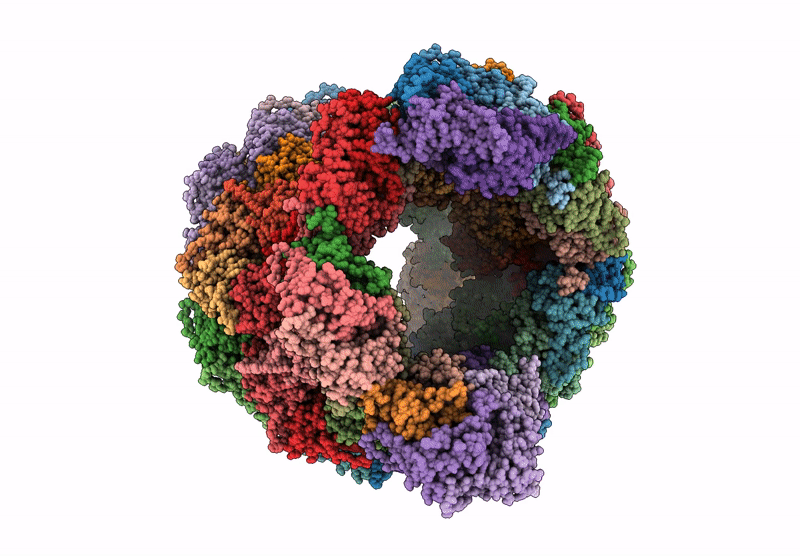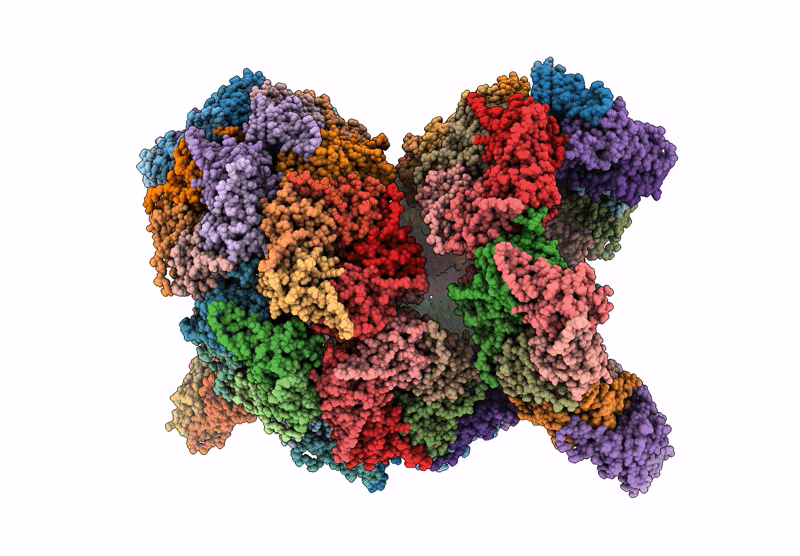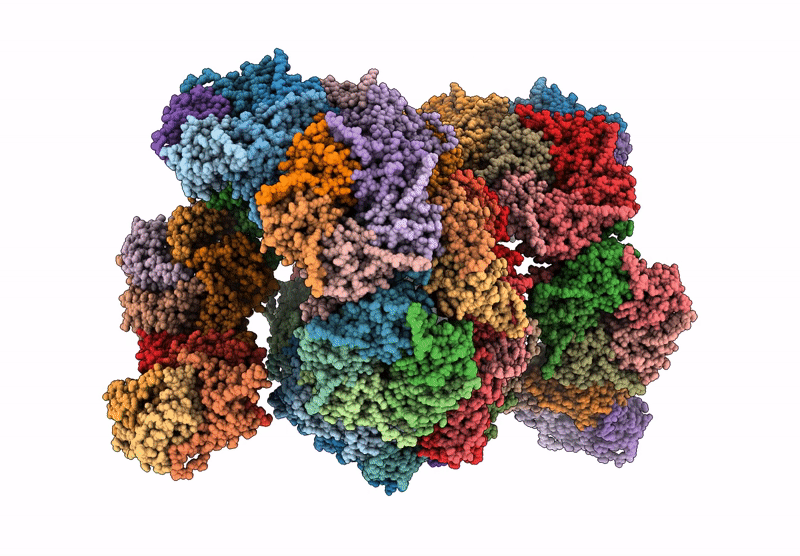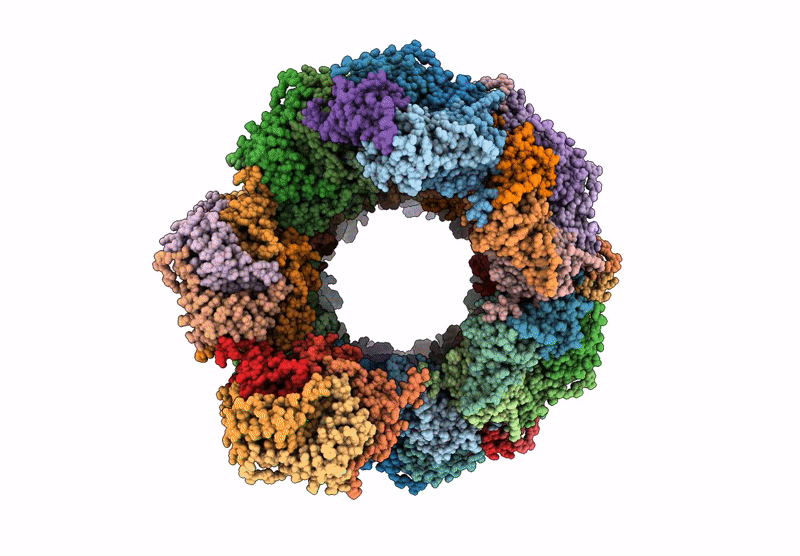
Deposition Date
2024-07-12
Release Date
2025-03-05
Last Version Date
2025-03-19
Entry Detail
PDB ID:
9G3I
Keywords:
Title:
Circularly permuted lumazine synthase twisted tube with 18 Angstrom gap between double strands
Biological Source:
Source Organism(s):
Aquifex aeolicus VF5 (Taxon ID: 224324)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
2.85 Å
Aggregation State:
HELICAL ARRAY
Reconstruction Method:
HELICAL